CARVEDILOL tablet, film coated
Carvedilol by
Drug Labeling and Warnings
Carvedilol by is a Prescription medication manufactured, distributed, or labeled by Bryant Ranch Prepack. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use CARVEDILOL TABLETS, USP safely and effectively. See full prescribing information for
CARVEDILOL TABLETS, USP, FILM COATED FOR ORAL USE
Initial U.S. Approval: 1995INDICATIONS AND USAGE
DOSAGE AND ADMINISTRATION
Take with food. Individualize dosage and monitor during up-titration. (2)
- Heart failure: Start at 3.125 mg twice daily and increase to 6.25, 12.5, and then 25 mg twice daily over intervals of at least 2 weeks. Maintain lower doses if higher doses are not tolerated. (2.1)
- Left ventricular dysfunction following myocardial infarction: Start at 6.25 mg twice daily and increase to 12.5 mg then 25 mg twice daily after intervals of 3 to 10 days. A lower starting dose or slower titration may be used. (2.2)
- Hypertension: Start at 6.25 mg twice daily and increase if needed for blood pressure control to 12.5 mg then 25 mg twice daily over intervals of 1 to 2 weeks. (2.3)
DOSAGE FORMS AND STRENGTHS
Tablets: 3.125, 6.25, 12.5, 25 mg (3)
CONTRAINDICATIONS
- Bronchial asthma or related bronchospastic conditions (4)
- Second- or third-degree AV block (4)
- Sick sinus syndrome (4)
- Severe bradycardia (unless permanent pacemaker in place) (4)
- Patients in cardiogenic shock or decompensated heart failure requiring the use of IV inotropic therapy. (4)
- Severe hepatic impairment (2.4, 4)
- History of serious hypersensitivity reaction (e.g., Stevens-Johnson syndrome, anaphylactic reaction, angioedema) to any component of this medication or other medications containing carvedilol. (4)
WARNINGS AND PRECAUTIONS
- Acute exacerbation of coronary artery disease upon cessation of therapy: Do not abruptly discontinue. (5.1)
- Bradycardia, hypotension, worsening heart failure/fluid retention may occur. Reduce the dose as needed. (5.2, 5.3, 5.4)
- Non-allergic bronchospasm (e.g., chronic bronchitis and emphysema): Avoid β-blockers. (4) However, if deemed necessary, use with caution and at lowest effective dose. (5.5)
- Diabetes: Monitor glucose as β-blockers may mask symptoms of hypoglycemia or worsen hyperglycemia. (5.6)
ADVERSE REACTIONS
Most common adverse events (6.1):
- Heart failure and Left ventricular dysfunction following myocardial infarction (≥10%): Dizziness, fatigue, hypotension, diarrhea, hyperglycemia, asthenia, bradycardia, weight increase.
- Hypertension (≥ 5%): Dizziness
To report SUSPECTED ADVERSE REACTIONS, contact Beximco Pharmaceuticals USA Inc. at 877-372-6093 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
DRUG INTERACTIONS
- CYP P450 2D6 enzyme inhibitors may increase and rifampin may decrease Carvedilol Tablet levels. (7.1, 7.5)
- Hypotensive agents (e.g., reserpine, MAO inhibitors, clonidine) may increase the risk of hypotension and/or severe bradycardia. (7.2)
- Cyclosporine or digoxin levels may increase. (7.3, 7.4)
- Both digitalis glycosides and β-blockers slow atrioventricular conduction and decrease heart rate. Concomitant use can increase the risk of bradycardia. (7.4)
- Amiodarone may increase Carvedilol Tablet levels resulting in further slowing of the heart rate or cardiac conduction. (7.6)
- Verapamil- or diltiazem-type calcium channel blockers may affect ECG and/or blood pressure. (7.7)
- Insulin and oral hypoglycemics action may be enhanced. (7.8)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 7/2024
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Heart Failure
1.2 Left Ventricular Dysfunction Following Myocardial Infarction
1.3 Hypertension
2 DOSAGE AND ADMINISTRATION
2.1 Heart Failure
2.2 Left Ventricular Dysfunction Following Myocardial Infarction
2.3 Hypertension
2.4 Hepatic Impairment
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Cessation of Therapy
5.2 Bradycardia
5.3 Hypotension
5.4 Heart Failure/Fluid Retention
5.5 Non-Allergic Bronchospasm
5.6 Glycemic Control in Type 2 Diabetes
5.7 Peripheral Vascular Disease
5.8 Deterioration of Renal Function
5.9 Major Surgery
5.10 Thyrotoxicosis
5.11 Pheochromocytoma
5.12 Prinzmetal's Variant Angina
5.13 Risk of Anaphylactic Reaction
5.14 Intraoperative Floppy Iris Syndrome
6 ADVERSE REACTIONS
6.1 Clinical Studies Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 CYP2D6 Inhibitors and Poor Metabolizers
7.2 Hypotensive Agents
7.3 Cyclosporine
7.4 Digitalis Glycosides
7.5 Inducers/Inhibitors of Hepatic Metabolism
7.6 Amiodarone
7.7 Calcium Channel Blockers
7.8 Insulin or Oral Hypoglycemics
7.9 Anesthesia
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
12.4 Specific Populations
12.5 Drug-Drug Interactions
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Heart Failure
14.2 Left Ventricular Dysfunction Following Myocardial Infarction
14.3 Hypertension
14.4 Hypertension With Type 2 Diabetes Mellitus
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
1.1 Heart Failure
Carvedilol Tablets is indicated for the treatment of mild-to-severe chronic heart failure of ischemic or cardiomyopathic origin, usually in addition to diuretics, ACE inhibitors, and digitalis, to increase survival and, also, to reduce the risk of hospitalization [see Drug Interactions (7.4), Clinical Studies (14.1)].
1.2 Left Ventricular Dysfunction Following Myocardial Infarction
Carvedilol Tablet is indicated to reduce cardiovascular mortality in clinically stable patients who have survived the acute phase of a myocardial infarction and have a left ventricular ejection fraction of less than or equal to 40% (with or without symptomatic heart failure) [see Clinical Studies (14.2)].
-
2 DOSAGE AND ADMINISTRATION
Carvedilol Tablet should be taken with food to slow the rate of absorption and reduce the incidence of orthostatic effects.
2.1 Heart Failure
DOSAGE MUST BE INDIVIDUALIZED AND CLOSELY MONITORED BY A PHYSICIAN DURING UP-TITRATION. Prior to initiation of Carvedilol Tablets, it is recommended that fluid retention be minimized. The recommended starting dose of Carvedilol Tablets is 3.125 mg twice daily for 2 weeks. If tolerated, patients may have their dose increased to 6.25, 12.5, and 25 mg twice daily over successive intervals of at least 2 weeks. Patients should be maintained on lower doses if higher doses are not tolerated. A maximum dose of 50 mg twice daily has been administered to patients with mild-to-moderate heart failure weighing over 85 kg (187 lbs.).
Patients should be advised that initiation of treatment and (to a lesser extent) dosage increases may be associated with transient symptoms of dizziness or lightheadedness (and rarely syncope) within the first hour after dosing. During these periods, patients should avoid situations such as driving or hazardous tasks, where symptoms could result in injury. Vasodilatory symptoms often do not require treatment, but it may be useful to separate the time of dosing of Carvedilol Tablets from that of the ACE inhibitor or to reduce temporarily the dose of the ACE inhibitor. The dose of Carvedilol Tablets should not be increased until symptoms of worsening heart failure or vasodilation have been stabilized. Fluid retention (with or without transient worsening heart failure symptoms) should be treated by an increase in the dose of diuretics. The dose of Carvedilol Tablets should be reduced if patients experience bradycardia (heart rate <55 beats/minute). Episodes of dizziness or fluid retention during initiation of Carvedilol Tablets can generally be managed without discontinuation of treatment and do not preclude subsequent successful titration of, or a favorable response to, carvedilol.
2.2 Left Ventricular Dysfunction Following Myocardial Infarction
DOSAGE MUST BE INDIVIDUALIZED AND MONITORED DURING UP-TITRATION. Treatment with Carvedilol Tablet may be started as an inpatient or outpatient and should be started after the patient is hemodynamically stable and fluid retention has been minimized. It is recommended that Carvedilol Tablets be started at 6.25 mg twice daily and increased after 3 to 10 days, based on tolerability, to 12.5 mg twice daily, then again to the target dose of 25 mg twice daily. A lower starting dose may be used (3.125 mg twice daily) and/or the rate of up-titration may be slowed if clinically indicated (e.g., due to low blood pressure or heart rate, or fluid retention). Patients should be maintained on lower doses if higher doses are not tolerated. The recommended dosing regimen need not be altered in patients who received treatment with an IV or oral β-blocker during the acute phase of the myocardial infarction.
2.3 Hypertension
DOSAGE MUST BE INDIVIDUALIZED. The recommended starting dose of Carvedilol Tablet is 6.25 mg twice daily. If this dose is tolerated, using standing systolic pressure measured about 1 hour after dosing as a guide, the dose should be maintained for 7 to 14 days, and then increased to 12.5 mg twice daily if needed, based on trough blood pressure, again using standing systolic pressure one hour after dosing as a guide for tolerance. This dose should also be maintained for 7 to 14 days and can then be adjusted upward to 25 mg twice daily if tolerated and needed. The full antihypertensive effect of Carvedilol Tablets is seen within 7 to 14 days. Total daily dose should not exceed 50 mg.
Concomitant administration with a diuretic can be expected to produce additive effects and exaggerate the orthostatic component of Carvedilol Tablet action.
2.4 Hepatic Impairment
Carvedilol Tablets should not be given to patients with severe hepatic impairment [see Contraindications (4)].
-
3 DOSAGE FORMS AND STRENGTHS
The white, oval shaped, film-coated tablets are available in the following strengths:
- 3.125 mg – White, oval shaped, film-coated tablet engraved with 254 on one side and plain on the other side,
- 6.25 mg – White, oval shaped, film-coated tablet engraved with 255 on one side and plain on the other side,
- 12.5 mg – White, oval shaped, film-coated tablet engraved with 256 on one side and plain on the other side, and
- 25 mg – White, oval shaped, film-coated tablet engraved with 257 on one side and plain on the other side.
-
4 CONTRAINDICATIONS
Carvedilol Tablet is contraindicated in the following conditions:
- Bronchial asthma or related bronchospastic conditions. Deaths from status asthmaticus have been reported following single doses of Carvedilol Tablet.
- Second- or third-degree AV block
- Sick sinus syndrome
- Severe bradycardia (unless a permanent pacemaker is in place)
- Patients with cardiogenic shock or who have decompensated heart failure requiring the use of intravenous inotropic therapy. Such patients should first be weaned from intravenous therapy before initiating Carvedilol Tablet.
- Patients with severe hepatic impairment
- Patients with a history of a serious hypersensitivity reaction (e.g., Stevens-Johnson syndrome, anaphylactic reaction, angioedema) to any component of this medication or other medications containing carvedilol.
-
5 WARNINGS AND PRECAUTIONS
5.1 Cessation of Therapy
Patients with coronary artery disease, who are being treated with Carvedilol Tablet should be advised against abrupt discontinuation of therapy. Severe exacerbation of angina and the occurrence of myocardial infarction and ventricular arrhythmias have been reported in angina patients following the abrupt discontinuation of therapy with β-blockers. The last 2 complications may occur with or without preceding exacerbation of the angina pectoris. As with other β-blockers, when discontinuation of Carvedilol Tablet is planned, the patients should be carefully observed and advised to limit physical activity to a minimum. Carvedilol Tablet should be discontinued over 1 to 2 weeks whenever possible. If the angina worsens or acute coronary insufficiency develops, it is recommended that Carvedilol Tablet be promptly reinstituted, at least temporarily. Because coronary artery disease is common and may be unrecognized, it may be prudent not to discontinue therapy with Carvedilol Tablet abruptly even in patients treated only for hypertension or heart failure.
5.2 Bradycardia
In clinical trials, Carvedilol Tablet caused bradycardia in about 2% of hypertensive patients, 9% of heart failure patients and 6.5% of myocardial infarction patients with left ventricular dysfunction. If pulse rate drops below 55 beats per minute, the dosage should be reduced.
5.3 Hypotension
In clinical trials of primarily mild-to-moderate heart failure, hypotension and postural hypotension occurred in 9.7% and syncope in 3.4% of subjects receiving Carvedilol Tablets compared with 3.6% and 2.5% of placebo subjects, respectively. The risk for these events was highest during the first 30 days of dosing, corresponding to the up-titration period and was a cause for discontinuation of therapy in 0.7% of subjects receiving Carvedilol Tablets, compared with 0.4% of placebo subjects. In a long-term, placebo-controlled trial in severe heart failure (COPERNICUS), hypotension and postural hypotension occurred in 15.1% and syncope in 2.9% of heart failure subjects receiving Carvedilol Tablets compared with 8.7% and 2.3% of placebo subjects, respectively. These events were a cause for discontinuation of therapy in 1.1% of subjects receiving Carvedilol Tablets, compared with 0.8% of placebo subjects.
Postural hypotension occurred in 1.8% and syncope in 0.1% of hypertensive patients, primarily following the initial dose or at the time of dose increase and was a cause for discontinuation of therapy in 1% of patients.
In the CAPRICORN study of survivors of an acute myocardial infarction, hypotension or postural hypotension occurred in 20.2% of patients receiving Carvedilol Tablet compared to 12.6% of placebo patients. Syncope was reported in 3.9% and 1.9% of patients, respectively. These events were a cause for discontinuation of therapy in 2.5% of patients receiving Carvedilol Tablet, compared to 0.2% of placebo patients.
Starting with a low dose, administration with food, and gradual up-titration should decrease the likelihood of syncope or excessive hypotension [see Dosage and Administration (2.1, 2.2, 2.3)]. During initiation of therapy, the patient should be cautioned to avoid situations such as driving or hazardous tasks, where injury could result should syncope occur.
5.4 Heart Failure/Fluid Retention
Worsening heart failure or fluid retention may occur during up-titration of Carvedilol Tablet. If such symptoms occur, diuretics should be increased and the Carvedilol Tablet dose should not be advanced until clinical stability resumes [see Dosage and Administration (2)]. Occasionally it is necessary to lower the Carvedilol Tablet dose or temporarily discontinue it. Such episodes do not preclude subsequent successful titration of, or a favorable response to carvedilol. In a placebo-controlled trial of subjects with severe heart failure, worsening heart failure during the first 3 months was reported to a similar degree with carvedilol and with placebo. When treatment was maintained beyond 3 months, worsening heart failure was reported less frequently in subjects treated with carvedilol than with placebo. Worsening heart failure observed during long-term therapy is more likely to be related to the patients’ underlying disease than to treatment with carvedilol.
5.5 Non-Allergic Bronchospasm
Patients with bronchospastic disease (e.g., chronic bronchitis and emphysema) should, in general, not receive β-blockers. Carvedilol Tablet may be used with caution, however, in patients who do not respond to, or cannot tolerate, other antihypertensive agents. It is prudent, if Carvedilol Tablet is used, to use the smallest effective dose, so that inhibition of endogenous or exogenous β-agonists is minimized.
In clinical trials of patients with heart failure, patients with bronchospastic disease were enrolled if they did not require oral or inhaled medication to treat their bronchospastic disease. In such patients, it is recommended that Carvedilol Tablet be used with caution. The dosing recommendations should be followed closely and the dose should be lowered if any evidence of bronchospasm is observed during up-titration.
5.6 Glycemic Control in Type 2 Diabetes
In general, β-blockers may mask some of the manifestations of hypoglycemia, particularly tachycardia. Nonselective β-blockers may potentiate insulin-induced hypoglycemia and delay recovery of serum glucose levels. Patients subject to spontaneous hypoglycemia, or diabetic patients receiving insulin or oral hypoglycemic agents, should be cautioned about these possibilities.
In heart failure patients with diabetes, carvedilol therapy may lead to worsening hyperglycemia, which responds to intensification of hypoglycemic therapy. It is recommended that blood glucose be monitored when carvedilol dosing is initiated, adjusted, or discontinued.
Studies designed to examine the effects of carvedilol on glycemic control in patients with diabetes and heart failure have not been conducted. In a study designed to examine the effects of carvedilol on glycemic control in a population with mild-to-moderate hypertension and well-controlled type 2 diabetes mellitus, carvedilol had no adverse effect on glycemic control, based on HbA1c measurements [see Clinical Studies (14.4)].
5.7 Peripheral Vascular Disease
β-blockers can precipitate or aggravate symptoms of arterial insufficiency in patients with peripheral vascular disease. Caution should be exercised in such individuals.
5.8 Deterioration of Renal Function
Rarely, use of Carvedilol Tablet in patients with heart failure has resulted in deterioration of renal function. Patients at risk appear to be those with low blood pressure (systolic blood pressure less than 100 mm Hg), ischemic heart disease and diffuse vascular disease, and/or underlying renal insufficiency. Renal function has returned to baseline when Carvedilol Tablet was stopped. In patients with these risk factors it is recommended that renal function be monitored during up-titration of Carvedilol Tablet and the drug discontinued or dosage reduced if worsening of renal function occurs.
5.9 Major Surgery
Chronically administered beta blocking therapy should not be routinely withdrawn prior to major surgery; however, the impaired ability of the heart to respond to reflex adrenergic stimuli may augment the risks of general anesthesia and surgical procedures.
5.10 Thyrotoxicosis
β-adrenergic blockade may mask clinical signs of hyperthyroidism, such as tachycardia. Abrupt withdrawal of β-blockade may be followed by an exacerbation of the symptoms of hyperthyroidism or may precipitate thyroid storm.
5.11 Pheochromocytoma
In patients with pheochromocytoma, an α-blocking agent should be initiated prior to the use of any β-blocking agent. Although Carvedilol Tablet has both α- and β-blocking pharmacologic activities, there has been no experience with its use in this condition. Therefore, caution should be taken in the administration of Carvedilol Tablet to patients suspected of having pheochromocytoma.
5.12 Prinzmetal's Variant Angina
Agents with non-selective β-blocking activity may provoke chest pain in patients with Prinzmetal's variant angina. There has been no clinical experience with Carvedilol Tablet in these patients although the α-blocking activity may prevent such symptoms. However, caution should be taken in the administration of Carvedilol Tablet to patients suspected of having Prinzmetal's variant angina.
5.13 Risk of Anaphylactic Reaction
While taking β-blockers, patients with a history of severe anaphylactic reaction to a variety of allergens may be more reactive to repeated challenge, either accidental, diagnostic, or therapeutic. Such patients may be unresponsive to the usual doses of epinephrine used to treat allergic reaction.
5.14 Intraoperative Floppy Iris Syndrome
Intraoperative Floppy Iris Syndrome (IFIS) has been observed during cataract surgery in some patients treated with α -1 blockers (Carvedilol Tablet is an alpha/beta blocker). This variant of small pupil syndrome is characterized by the combination of a flaccid iris that billows in response to intraoperative irrigation currents, progressive intraoperative miosis despite preoperative dilation with standard mydriatic drugs, and potential prolapse of the iris toward the phacoemulsification incisions. The patient’s ophthalmologist should be prepared for possible modifications to the surgical technique, such as utilization of iris hooks, iris dilator rings, or viscoelastic substances. There does not appear to be a benefit of stopping alpha-1 blocker therapy prior to cataract surgery.
-
6 ADVERSE REACTIONS
6.1 Clinical Studies Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared with rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Carvedilol Tablet has been evaluated for safety in patients with heart failure (mild, moderate and severe), in patients with left ventricular dysfunction following myocardial infarction and in hypertensive patients. The observed adverse event profile was consistent with the pharmacology of the drug and the health status of the patients in the clinical trials. Adverse events reported for each of these patient populations are provided below. Excluded are adverse events considered too general to be informative, and those not reasonably associated with the use of the drug because they were associated with the condition being treated or are very common in the treated population. Rates of adverse events were generally similar across demographic subsets (men and women, elderly and non-elderly, blacks and non-blacks).
Heart Failure
Carvedilol Tablets has been evaluated for safety in heart failure in more than 4,500 subjects worldwide of whom more than 2,100 participated in placebo-controlled clinical trials. Approximately 60% of the total treated population in placebo-controlled clinical trials received Carvedilol Tablets for at least 6 months and 30% received Carvedilol Tablets for at least 12 months. In the COMET trial, 1,511 subjects with mild-to-moderate heart failure were treated with Carvedilol Tablets for up to 5.9 years (mean: 4.8 years). Both in US clinical trials in mild-to-moderate heart failure that compared Carvedilol Tablets in daily doses up to 100 mg (n = 765) with placebo (n = 437), and in a multinational clinical trial in severe heart failure (COPERNICUS) that compared Carvedilol Tablets in daily doses up to 50 mg (n = 1,156) with placebo (n = 1,133), discontinuation rates for adverse experiences were similar in carvedilol and placebo subjects. In placebo-controlled clinical trials, the only cause of discontinuation >1%, and occurring more often on carvedilol was dizziness (1.3% on carvedilol, 0.6% on placebo in the COPERNICUS trial). Table 1 shows adverse events reported in subjects with mild-to-moderate heart failure enrolled in US placebo-controlled clinical trials, and with severe heart failure enrolled in the COPERNICUS trial. Shown are adverse events that occurred more frequently in drug-treated subjects than placebo-treated subjects with an incidence of >3% in subjects treated with carvedilol regardless of causality. Median trial medication exposure was 6.3 months for both carvedilol and placebo subjects in the trials of mild-to-moderate heart failure, and 10.4 months in the trial of severe heart failure subjects. The adverse event profile of Carvedilol Tablets observed in the long-term COMET trial was generally similar to that observed in the US Heart Failure Trials.
Table 1. Adverse Events (%) Occurring More Frequently with Carvedilol Tablet than with
Placebo in Subjects with Mild-to-Moderate Heart Failure (HF) Enrolled in US Heart Failure Trials or in Subjects with Severe Heart Failure in the COPERNICUS Trial
(Incidence >3% in Subjects Treated with Carvedilol, Regardless of Causality)
Body System/
Adverse Event
Mild-to-Moderate HF
Severe HF
Carvedilol Tablets
Placebo
Carvedilol Tablets
Placebo
(n = 765)
(n = 437)
(n = 1,156)
(n = 1,133)
Body as a Whole
Asthenia
7
7
11
9
Fatigue
24
22
-
-
Digoxin level increased
5
4
2
1
Edema generalized
5
3
6
5
Edema dependent
4
2
-
-
Cardiovascular
Bradycardia
9
1
10
3
Hypotension
9
3
14
8
Syncope
3
3
8
5
Angina pectoris
2
3
6
4
Central Nervous System
Dizziness
32
19
24
17
Headache
8
7
5
3
Gastrointestinal
Diarrhea
12
6
5
3
Nausea
9
5
4
3
Vomiting
6
4
1
2
Metabolic
Hyperglycemia
12
8
5
3
Weight increase
10
7
12
11
BUN increased
6
5
-
-
NPN increased
6
5
-
-
Hypercholesterolemia
4
3
1
1
Edema peripheral
2
1
7
6
Musculoskeletal
Arthralgia
6
5
1
1
Respiratory
Cough increased
8
9
5
4
Rales
4
4
4
2
Vision
Vision abnormal
5
2
-
-
Cardiac failure and dyspnea were also reported in these trials, but the rates were equal or greater in subjects who received placebo. The following adverse events were reported with a frequency of greater than 1% but less than or equal to 3% and more frequently with Carvedilol Tablets in either the US placebo-controlled trials in subjects with mild-to-moderate heart failure, or in subjects with severe heart failure in the COPERNICUS trial.
Incidence greater than 1% to less than or equal to 3%
Body as a Whole: Allergy, malaise, hypovolemia, fever, leg edema.
Cardiovascular: Fluid overload, postural hypotension, aggravated angina pectoris, AV block, palpitation, hypertension.
Central and Peripheral Nervous System: Hypesthesia, vertigo, paresthesia.
Gastrointestinal: Melena, periodontitis.
Liver and Biliary System: SGPT increased, SGOT increased.
Metabolic and Nutritional: Hyperuricemia, hypoglycemia, hyponatremia, increased alkaline phosphatase, glycosuria, hypervolemia, diabetes mellitus, GGT increased, weight loss, hyperkalemia, creatinine increased.
Musculoskeletal: Muscle cramps.
Platelet, Bleeding and Clotting: Prothrombin decreased, purpura, thrombocyt penia.
Psychiatric: Somnolence.
Reproductive, male: Impotence.
Special Senses: Blurred vision.
Urinary System: Renal insufficiency, albuminuria, hematuria.
Left Ventricular Dysfunction Following Myocardial Infarction:
Carvedilol Tablet has been evaluated for safety in survivors of an acute myocardial infarction with left ventricular dysfunction in the CAPRICORN trial which involved 969 subjects who received Carvedilol Tablet and 980 who received placebo. Approximately 75% of the subjects received Carvedilol Tablet for at least 6 months and 53% received Carvedilol Tablet for at least 12 months. Subjects were treated for an average of 12.9 months and 12.8 months with Carvedilol Tablet and placebo, respectively.
The most common adverse events reported with Carvedilol Tablets in the CAPRICORN trial were consistent with the profile of the drug in the US heart failure trials and the COPERNICUS trial. The only additional adverse events reported in CAPRICORN in greater than 3% of the subjects and more commonly on carvedilol were dyspnea, anemia, and lung edema.
The following adverse events were reported with a frequency of greater than 1% but less than or equal to 3% and more frequently with Carvedilol Tablet: Flu syndrome, cerebrovascular accident, peripheral vascular disorder, hypotonia, depression, gastrointestinal pain, arthritis, and gout. The overall rates of discontinuations due to adverse events were similar in both groups of patients. In this database, the only cause of discontinuation greater than 1%, and occurring more often on Carvedilol Tablet was hypotension (1.5% on Carvedilol Tablet, 0.2% on placebo).
Hypertension:
Carvedilol Tablet has been evaluated for safety in hypertension in more than 2,193 subjects in U.S. clinical trials and in 2,976 subjects in international clinical trials. Approximately 36% of the total treated population received Carvedilol Tablet for at least 6 months. Most adverse events reported during therapy with Carvedilol Tablet were of mild to moderate severity. In U.S. controlled clinical trials directly comparing Carvedilol Tablet in doses up to 50 mg (n = 1,142) to placebo (n = 462), 4.9% of subjects receiving Carvedilol Tablet discontinued for adverse events versus 5.2% of placebo subjects. Although there was no overall difference in discontinuation rates, discontinuations were more common in the Carvedilol Tablet group for postural hypotension (1% versus 0). The overall incidence of adverse events in U.S. placebo-controlled trials increased with increasing dose of Carvedilol Tablet. For individual adverse events this could only be distinguished for dizziness, which increased in frequency from 2% to 5% as total daily dose increased from 6.25 mg to 50 mg.
Table 2 shows adverse events in U.S. placebo-controlled clinical trials for hypertension that occurred with an incidence of greater than or equal to 1% regardless of causality, and that were more frequent in drug-treated subjects than placebo-treated patients.
Table 2. Adverse Events (%) Occurring in U.S. Placebo-Controlled
Hypertension Trials (Incidence greater than or equal to 1%, Regardless of Causality)*
Body system/ Adverse Event
Carvedilol Tablet (n = 1,142)
Placebo (n = 462)
Cardiovascular
Bradycardia
2
-
Postural hypotension
2
-
Peripheral edema
1
-
Central Nervous System
Dizziness
6
5
Insomnia
2
1
Gastrointestinal
Diarrhea
2
1
Hematologic
Thrombocytopenia
1
-
Metabolic
Hypertriglyceridemia
1
-
*Shown are events with rate greater than 1% rounded to nearest integer.
Dyspnea and fatigue were also reported in these studies, but the rates were equal or greater in patients who received placebo.
The following adverse events not described above were reported as possibly or probably related to Carvedilol Tablet in worldwide open or controlled trials with Carvedilol Tablet in patients with hypertension or heart failure.
Incidence greater than 0.1% to less than or equal to 1%
Cardiovascular: Peripheral ischemia, tachycardia.
Central and Peripheral Nervous System: Hypokinesia.
Gastrointestinal: Bilirubinemia, increased hepatic enzymes (0.2% of hypertension patients and 0.4% of heart failure patients were discontinued from therapy because of increases in hepatic enzymes) [see Adverse Reactions (6.2)].
Psychiatric: Nervousness, sleep disorder, aggravated depression, impaired concentration, abnormal thinking, paroniria, emotional lability.
Respiratory System: Asthma [see Contraindications (4)].
Reproductive, male: Decreased libido.
Skin and Appendages: Pruritus, rash erythematous, rash maculopapular, rash psoriaform, photosensitivity reaction.
Special Senses: Tinnitus.
Urinary System: Micturition frequency increased.
Autonomic Nervous System: Dry mouth, sweating increased.
Metabolic and Nutritional: Hypokalemia, hypertriglyceridemia.
Hematologic: Anemia, leukopenia.
The following events were reported in less than or equal to 0.1% of patients and are potentially important: Complete AV block, bundle branch block, myocardial ischemia, cerebrovascular disorder, convulsions, migraine, neuralgia, paresis, anaphylactoid reaction, alopecia, exfoliative dermatitis, amnesia, GI hemorrhage, bronchospasm, pulmonary edema, decreased hearing, respiratory alkalosis, increased BUN, decreased HDL, pancytopenia, and atypical lymphocytes.
Laboratory Abnormalities
Reversible elevations in serum transaminases (ALT or AST) have been observed during treatment with Carvedilol Tablet. Rates of transaminase elevations (2 to 3 times the upper limit of normal) observed during controlled clinical trials have generally been similar between subjects treated with Carvedilol Tablet and those treated with placebo. However, transaminase elevations, confirmed by re-challenge, have been observed with Carvedilol Tablet. In a long-term, placebo-controlled trial in severe heart failure, subjects treated with Carvedilol Tablet had lower values for hepatic transaminases than subjects treated with placebo, possibly because improvements in cardiac function induced by Carvedilol Tablet led to less hepatic congestion and/or improved hepatic blood flow.
Carvedilol Tablet has not been associated with clinically significant changes in serum potassium, total triglycerides, total cholesterol, HDL, Uric acid, blood urea nitrogen, or creatinine. No clinically relevant changes were noted in fasting serum glucose in hypertensive patients; fasting serum glucose was not evaluated in the heart failure clinical trials.6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of Carvedilol Tablet. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Blood and Lymphatic System Disorders: Aplastic anemia.
Immune System Disorders: Hypersensitivity (e.g., anaphylactic reactions, angioedema, urticaria).
Renal and Urinary Disorders: Urinary incontinence.
Respiratory, Thoracic and Mediastinal Disorders: Interstitial pneumonitis.
Skin and Subcutaneous Tissue Disorders: Stevens-Johnson syndrome, toxic epidermal necrolysis, erythema multiforme.
-
7 DRUG INTERACTIONS
7.1 CYP2D6 Inhibitors and Poor Metabolizers
Interactions of Carvedilol Tablet with potent inhibitors of CYP2D6 isoenzyme (such as quinidine, fluoxetine, paroxetine, and propafenone) have not been studied, but these drugs would be expected to increase blood levels of the R(+) enantiomer of Carvedilol Tablet [see Clinical Pharmacology (12.3)]. Retrospective analysis of side effects in clinical trials showed that poor 2D6 metabolizers had a higher rate of dizziness during uptitration, presumably resulting from vasodilating effects of the higher concentrations of the α-blocking R(+) enantiomer.
7.2 Hypotensive Agents
Patients taking both agents with β-blocking properties and a drug that can deplete catecholamines (e.g., reserpine and monoamine oxidase inhibitors) should be observed closely for signs of hypotension and/or severe bradycardia.
Concomitant administration of clonidine with agents with β-blocking properties may may cause hypotension and bradycardia. When concomitant treatment with agents with β-blocking properties and clonidine is to be terminated, the β-blocking agent should be discontinued first. Clonidine therapy can then be discontinued several days later by gradually decreasing the dosage.
7.3 Cyclosporine
Modest increases in mean trough cyclosporine concentrations were observed following initiation of Carvedilol Tablet treatment in 21 renal transplant patients suffering from chronic vascular rejection. In about 30% of patients, the dose of cyclosporine had to be reduced in order to maintain cyclosporine concentrations within the therapeutic range, while in the remainder no adjustment was needed. On the average for the group, the dose of cyclosporine was reduced about 20% in these patients. Due to wide interindividual variability in the dose adjustment required, it is recommended that cyclosporine concentrations be monitored closely after initiation of Carvedilol Tablet therapy and that the dose of cyclosporine be adjusted as appropriate.
7.4 Digitalis Glycosides
Both digitalis glycosides and β-blockers slow atrioventricular conduction and decrease heart rate. Concomitant use can increase the risk of bradycardia. Digoxin concentrations are increased by about 15% when digoxin and Carvedilol Tablet are administered concomitantly. Therefore, increased monitoring of digoxin is recommended when initiating, adjusting, or discontinuing Carvedilol Tablet [see Clinical Pharmacology (12.5)].
7.5 Inducers/Inhibitors of Hepatic Metabolism
Rifampin reduced plasma concentrations of Carvedilol Tablet by about 70% [see Clinical Pharmacology (12.5)]. Cimetidine increased AUC by about 30% but caused no change in Cmax [see Clinical Pharmacology (12.5)].
7.6 Amiodarone
Amiodarone, and its metabolite desethyl amiodarone, inhibitors of CYP2C9 and P-glycoprotein, increased concentrations of the S(-)-enantiomer of Carvedilol Tablet by at least 2-fold [see Clinical Pharmacology (12.5)]. The concomitant administration of amiodarone or other CYP2C9 inhibitors such as fluconazole with Carvedilol Tablet may enhance the β-blocking properties of Carvedilol Tablet resulting in further slowing of the heart rate or cardiac conduction. Patients should be observed for signs of bradycardia or heart block, particularly when one agent is added to preexisting treatment with the other.
7.7 Calcium Channel Blockers
Conduction disturbance (rarely with hemodynamic compromise) has been observed when Carvedilol Tablets is coadministered with diltiazem. As with other agents with β-blocking properties, if Carvedilol Tablets is to be administered with calcium channel blockers of the verapamil or diltiazem type, it is recommended that ECG and blood pressure be monitored.
7.8 Insulin or Oral Hypoglycemics
Agents with β-blocking properties may enhance the blood-sugar-reducing effect of insulin and oral hypoglycemics. Therefore, in patients taking insulin or oral hypoglycemics, regular monitoring of blood glucose is recommended [see Warnings and Precautions (5.6)].
7.9 Anesthesia
If treatment with Carvedilol Tablet is to be continued perioperatively, particular care should be taken when anesthetic agents which depress myocardial function, such as ether,cyclopropane, and trichloroethylene, are used [see Overdosage (10)].
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Available data regarding use of carvedilol in pregnant women are insufficient to determine whether there are drug-associated risks of adverse developmental outcomes. There are risks to the mother and fetus associated with poorly controlled hypertension in pregnancy. The use of beta blockers during the third trimester of pregnancy may increase the risk of hypotension, bradycardia, hypoglycemia, and Respiratory depression in the neonate [see Clinical Considerations]. In animal reproduction studies, there was no evidence of adverse developmental outcomes at clinically relevant doses [see Data]. Oral administration of carvedilol to pregnant rats during organogenesis resulted in post-implantation loss, decreased fetal body weight, and an increased frequency of delayed fetal skeletal development at maternally toxic doses that were 50 times the maximum recommended human dose (MRHD). In addition, oral administration of carvedilol to pregnant rabbits during organogenesis resulted in increased post-implantation loss at doses 25 times the MRHD [see Data]. The estimated background risk of major birth defects and miscarriage for the indicated populations are unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.Clinical Considerations
Disease-Associated Maternal and/or Embryo/Fetal Risk: Hypertension in pregnancy increases the maternal risk for pre-eclampsia, gestational diabetes, premature delivery, and delivery complications (e.g., need for cesarean section and post-partum hemorrhage). Hypertension increases the fetal risk for intrauterine growth restriction and intrauterine death. Pregnant women with hypertension should be carefully monitored and managed accordingly.
Fetal/Neonatal Adverse Reactions: Neonates of women with hypertension who are treated with beta-blockers during the third trimester of pregnancy may be at increased risk for hypotension, bradycardia, hypoglycemia, and respiratory depression. Observe newborns for symptoms of hypotension, bradycardia, hypoglycemia, and respiratory depression and manage accordingly.Data
Animal Data: Studies performed in rats and rabbits given carvedilol during fetal organogenesis revealed increased post-implantation loss in rats at a maternally toxic dose of 300 mg per kg per day (50 times the MRHD as mg per m2) and in rabbits (in the absence of maternal toxicity) at doses of 75 mg per kg per day (25 times the MRHD as mg per m2). In the rats, there was also a decrease in fetal body weight at 300 mg per kg per day (50 times the MRHD as mg per m’) accompanied by an increased incidence of fetuses with delayed skeletal development. In rats, the no-effect level for embryo-fetal toxicity was 60 mg per kg per day (10 times the MRHD as mg per m2; in rabbits, it was 15 mg per kg per day (5 times the MRHD as mg per m2). In a pre-and post-natal development study in rats administered carvedilol from late gestation through lactation, increased embryo-lethality was observed at a maternally toxic dose of 200 mg per kg per day (approximately 32 times the MRHD as mg per m2), and pup mortality and delays in physical growth/development were observed at 60 mg per kg per day (10 times the MRHD as mg per m2) in the absence of maternal toxicity. The no-effect level was 12 mg per kg per day (2 times the MRHD as mg per m2). Carvedilol was present in fetal rat tissue.8.2 Lactation
Risk Summary
There are no data on the presence of carvedilol in human milk, the effects on the breastfed infant, or the effects on milk production. Carvedilol is present in the milk of lactating rats. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for carvedilol and any potential adverse effects on the breastfed infant from carvedilol or from the underlying maternal condition.8.4 Pediatric Use
Effectiveness of Carvedilol Tablets in patients younger than 18 years of age has not been established. In a double-blind trial, 161 children (mean age 6 years, range 2 months to 17 years; 45% less than 2 years old) with chronic heart failure [NYHA class II to IV, left ventricular ejection fraction less than 40% for children with a systemic left ventricle (LV), and moderate-severe ventricular dysfunction qualitatively by echo for those with a systemic ventricle that was not an LV] who were receiving standard background treatment were randomized to placebo or to two dose levels of Carvedilol Tablet. These dose levels produced placebo-corrected heart rate reduction of 4 to 6 heartbeats per minute, indicative of β- blockade activity. Exposure appeared to be lower in pediatric subjects than adults. After 8 months of follow-up, there was no significant effect of treatment on clinical outcomes. Adverse reactions in this trial that occurred in greater than 10% of patients treated with Carvedilol Tablet and at twice the rate of placebo-treated patients included chest pain (17% versus 6%), dizziness (13% versus 2%), and dyspnea (11% versus 0%).
8.5 Geriatric Use
Of the 765 subjects with heart failure randomized to Carvedilol Tablets in US clinical trials, 31% (235) were 65 years of age or older, and 7.3% (56) were 75 years of age or older. Of the 1,156 subjects randomized to Carvedilol Tablets in a long-term, placebo-controlled trial in severe heart failure, 47% (547) were 65 years of age or older, and 15% (174) were 75 years of age or older. Of 3,025 subjects receiving Carvedilol Tablets in heart failure trials worldwide, 42% were 65 years of age or older.
Of the 975 myocardial infarction patients randomized to Carvedilol Tablet in the CAPRICORN trial, 48% (468) were 65 years of age or older, and 11% (111) were 75 years of age or older.
Of the 2,065 hypertensive patients in U.S. clinical trials of efficacy or safety who were treated with Carvedilol Tablet, 21% (436) were 65 years of age or older. Of 3,722 patients receiving Carvedilol Tablet in hypertension clinical trials conducted worldwide, 24% were 65 years of age or older.
With the exception of dizziness in hypertensive patients (incidence 8.8% in the elderly versus 6% in younger patients), no overall differences in the safety or effectiveness (see Figure 2 and 4) were observed between the older subjects and younger subjects in each of these populations. Similarly, other reported clinical experience has not identified differences in responses between the elderly and younger subjects, but greater sensitivity of some older individuals cannot be ruled out.
-
10 OVERDOSAGE
Overdosage may cause severe hypotension, bradycardia, cardiac insufficiency, cardiogenic shock, and cardiac arrest. Respiratory problems, bronchospasms, vomiting, lapses of consciousness, and generalized seizures may also occur.
The patient should be placed in a supine position and, where necessary, kept under observation and treated under intensive-care conditions.
The following agents may be administered:
for excessive bradycardia: Atropine, 2 mg IV.
to support cardiovascular function: Glucagon, 5 to 10 mg IV rapidly over 30 seconds, followed by a continuous infusion of 5 mg/hour; sympathomimetics (dobutamine, isoprenaline, adrenaline) at doses according to body weight and effect.
If peripheral vasodilation dominates, it may be necessary to administer adrenaline or noradrenaline with continuous monitoring of circulatory conditions. For therapy-resistant bradycardia, pacemaker therapy should be performed. For bronchospasm, β-sympathomimetics (as aerosol or IV) or aminophylline IV should be given. In the event of seizures, slow IV injection of diazepam or clonazepam is recommended.
NOTE: In the event of severe intoxication where there are symptoms of shock, treatment with antidotes must be continued for a sufficiently long period of time consistent with the 7 to 10 hour half-life of carvedilol.
Cases of overdosage with Carvedilol Tablet alone or in combination with other drugs have been reported. Quantities ingested in some cases exceeded 1,000 milligrams. Symptoms experienced included low blood pressure and heart rate. Standard supportive treatment was provided and individuals recovered.
-
11 DESCRIPTION
Carvedilol is a nonselective β-adrenergic blocking agent with α1-blocking activity. It is (±)-1-(carbazol-4-yloxy)-3-[[2-(o-methoxyphenoxy)ethyl]amino]-2-propanol. Carvedilol Tablet is a racemic mixture with the following structure:
Carvedilol is a white, oval-shaped, biconvex film-coated tablet containing 3.125 mg, 6.25 mg, 12.5 mg, or 25 mg of Carvedilol Tablet. Inactive ingredients consist of colloidal silicon dioxide, crospovidone, hypromellose, lactose monohydrate, magnesium stearate, microcrystalline cellulose, polyethylene glycol, sodium citrate dihydrate, sucrose, and titanium dioxide.
Carvedilol Tablet is a white to off-white powder with a molecular weight of 406.5 and molecular formula of C24H26N2O4. It is freely soluble in dimethylsulfoxide; soluble in methylene chloride and methanol; sparingly soluble in 95% ethanol and isopropanol; slightly soluble in ethyl ether; and practically insoluble in water, gastric fluid (simulated, TS, pH 1.1), and intestinal fluid (simulated, TS without pancreatin, pH 7.5).
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Carvedilol Tablet is a racemic mixture in which nonselective β-adrenoreceptor blocking activity is present in the S(-) enantiomer and α1-adrenergic blocking activity is present in both R(+) and S(-) enantiomers at equal potency. Carvedilol Tablet has no intrinsic sympathomimetic activity.
12.2 Pharmacodynamics
Heart Failure
The basis for the beneficial effects of Carvedilol Tablets in heart failure is not established. Two placebo-controlled trials compared the acute hemodynamic effects of Carvedilol Tablets with baseline measurements in 59 and 49 subjects with NYHA class II-IV heart failure receiving diuretics, ACE inhibitors, and digitalis. There were significant reductions in systemic blood pressure, pulmonary artery pressure, pulmonary capillary wedge pressure, and heart rate. Initial effects on cardiac output, stroke volume index, and systemic vascular resistance were small and variable. These trials measured hemodynamic effects again at 12 to 14 weeks.
Carvedilol Tablets significantly reduced systemic blood pressure, pulmonary artery pressure, right atrial pressure, systemic vascular resistance, and heart rate, while stroke volume index was increased. Among 839 subjects with NYHA class II-III heart failure treated for 26 to 52 weeks in 4 US placebo-controlled trials, average left ventricular ejection fraction (EF) measured by radionuclide ventriculography increased by 9 EF units (%) in subjects receiving
Carvedilol Tablets and by 2 EF units in placebo subjects at a target dose of 25 to 50 mg twice daily. The effects of carvedilol on ejection fraction were related to dose. Doses of 6.25 mg twice daily, 12.5 mg twice daily, and 25 mg twice daily were associated with placebo-corrected increases in EF of 5 EF units, 6 EF units, and 8 EF units, respectively; each of these effects were nominally statistically significant.
Left Ventricular Dysfunction Following Myocardial Infarction
The basis for the beneficial effects of Carvedilol Tablet in patients with left ventricular dysfunction following an acute myocardial infarction is not established.
Hypertension
The mechanism by which β-blockade produces an antihypertensive effect has not been established. β-adrenoreceptor blocking activity has been demonstrated in animal and human studies showing that carvedilol (1) reduces cardiac output in normal subjects; (2) reduces exercise- and/or isoproterenol-induced tachycardia; and (3) reduces reflex orthostatic tachycardia. Significant β-adrenoreceptor blocking effect is usually seen within 1 hour of drug administration. α1-adrenoreceptor blocking activity has been demonstrated in human and animal studies, showing that carvedilol (1) attenuates the pressor effects of phenylephrine; (2) causes vasodilation; and (3) reduces peripheral vascular resistance. These effects contribute to the reduction of blood pressure and usually are seen within 30 minutes of drug administration.
Due to the α1-receptor blocking activity of carvedilol, blood pressure is lowered more in the standing than in the supine position, and symptoms of postural hypotension (1.8%), including rare instances of syncope, can occur. Following oral administration, when postural hypotension has occurred, it has been transient and is uncommon when Carvedilol Tablet is administered with food at the recommended starting dose and titration increments are closely followed [see Dosage and Administration (2)].
In hypertensive patients with normal renal function, therapeutic doses of Carvedilol Tablet decreased renal vascular resistance with no change in glomerular filtration rate or renal plasma flow. Changes in excretion of sodium, potassium, uric acid, and phosphorus in hypertensive patients with normal renal function were similar after Carvedilol Tablet and placebo.
Carvedilol Tablet has little effect on plasma catecholamines, plasma aldosterone, or electrolyte levels, but it does significantly reduce plasma renin activity when given for at least 4 weeks. It also increases levels of atrial natriuretic peptide.
12.3 Pharmacokinetics
Carvedilol Tablet is rapidly and extensively absorbed following oral administration, with absolute bioavailability of approximately 25% to 35% due to a significant degree of first pass metabolism. Following oral administration, the apparent mean terminal elimination half-life of carvedilol generally ranges from 7 to 10 hours. Plasma concentrations achieved are proportional to the oral dose administered. When administered with food, the rate of absorption is slowed, as evidenced by a delay in the time to reach peak plasma levels, with no significant difference in extent of bioavailability. Taking Carvedilol Tablet with food should minimize the risk of orthostatic hypotension.
Carvedilol is extensively metabolized. Following oral administration of radiolabelled carvedilol to healthy volunteers, carvedilol accounted for only about 7% of the total radioactivity in plasma as measured by area under the curve (AUC). Less than 2% of the dose was excreted unchanged in the urine. Carvedilol is metabolized primarily by aromatic ring oxidation and glucuronidation. The oxidative metabolites are further metabolized by conjugation via glucuronidation and sulfation. The metabolites of carvedilol are excreted primarily via the bile into the feces. Demethylation and hydroxylation at the phenol ring produce 3 active metabolites with β-receptor blocking activity. Based on preclinical studies, the 4’-hydroxyphenyl metabolite is approximately 13 times more potent than carvedilol for β-blockade.
Compared to carvedilol, the three active metabolites exhibit weak vasodilating activity. Plasma concentrations of the active metabolites are about one-tenth of those observed for carvedilol and have pharmacokinetics similar to the parent.
Carvedilol undergoes stereoselective first-pass metabolism with plasma levels of R (+)-Carvedilol approximately 2 to 3 times higher than S (-)-Carvedilol following oral administration in healthy subjects. The mean apparent terminal elimination half-lives for R(+)-Carvedilol range from 5 to 9 hours compared with 7 to 11 hours for the S(-)- enantiomer.
The primary P450 enzymes responsible for the metabolism of both R (+) and S (-)-Carvedilol in human liver microsomes were CYP2D6 and CYP2C9 and to a lesser extent CYP3A4, 2C19, 1A2, and 2E1. CYP2D6 is thought to be the major enzyme in the 4’- and 5’-hydroxylation of carvedilol, with a potential contribution from 3A4. CYP2C9 is thought to be of primary importance in the O-methylation pathway of S (-)-carvedilol.
Carvedilol is subject to the effects of genetic polymorphism with poor metabolizers of debrisoquin (a marker for cytochrome P450 2D6) exhibiting 2 to 3 fold higher plasma concentrations of R (+)-carvedilol compared to extensive metabolizers. In contrast, plasma levels of S (-)-carvedilol are increased only about 20% to 25% in poor metabolizers, indicating this enantiomer is metabolized to a lesser extent by cytochrome P450 2D6 than R (+)-carvedilol. The pharmacokinetics of carvedilol do not appear to be different in poor metabolizers of S-mephenytoin (patients deficient in cytochrome P450 2C19).
Carvedilol is more than 98% bound to plasma proteins, primarily with albumin. The plasma-protein binding is independent of concentration over the therapeutic range. Carvedilol is a basic, lipophilic compound with a steady-state volume of distribution of approximately 115 L, indicating substantial distribution into extravascular tissues. Plasma clearance ranges from 500 to 700 mL/min.
12.4 Specific Populations
Heart Failure
Steady-state plasma concentrations of carvedilol and its enantiomers increased proportionally over the 6.25 to 50 mg dose range in subjects with heart failure. Compared with healthy subjects, heart failure subjects had increased mean AUC and Cmax values for carvedilol and its enantiomers, with up to 50% to 100% higher values observed in 6 subjects with NYHA class IV heart failure. The mean apparent terminal elimination half-life for carvedilol was similar to that observed in healthy subjects.
Geriatric
Plasma levels of carvedilol average about 50% higher in the elderly compared to young subjects.
Hepatic Impairment
Compared to healthy subjects, patients with severe liver impairment (cirrhosis) exhibit a 4 to 7 fold increase in carvedilol levels. Carvedilol is contraindicated in patients with severe liver impairment.
Renal Impairment
Although carvedilol is metabolized primarily by the liver, plasma concentrations of carvedilol have been reported to be increased in patients with renal impairment. Based on mean AUC data, approximately 40% to 50% higher plasma concentrations of carvedilol were observed in hypertensive patients with moderate to severe renal impairment compared with a control group of hypertensive patients with normal renal function. However, the ranges of AUC values were similar for both groups. Changes in mean peak plasma levels were less pronounced, approximately 12% to 26% higher in patients with impaired renal function.
Consistent with its high degree of plasma protein-binding, carvedilol does not appear to be cleared significantly by hemodialysis.
12.5 Drug-Drug Interactions
Since carvedilol undergoes substantial oxidative metabolism, the metabolism and pharmacokinetics of carvedilol may be affected by induction or inhibition of cytochrome P450 enzymes.
Amiodarone
In a pharmacokinetic study conducted in 106 Japanese patients with heart failure, co-administration of small loading and maintenance doses of amiodarone with carvedilol resulted in at least a 2 fold increase in the steady-state trough concentrations of S(-)-carvedilol [see Drug Interactions (7.6)].
Cimetidine
In a pharmacokinetic study conducted in 10 healthy male subjects, cimetidine (1,000 mg/day) increased the steady state AUC of Carvedilol Tablet by 30% with no change in Cmax [see Drug Interactions (7.5)].
Digoxin
Following concomitant administration of carvedilol (25 mg once daily) and digoxin (0.25 mg once daily) for 14 days, steady-state AUC and trough concentrations of digoxin were increased by 14% and 16%, respectively, in 12 hypertensive patients [see Drug Interactions (7.4)].
Glyburide
In 12 healthy subjects, combined administration of carvedilol (25 mg once daily) and a single dose of glyburide did not result in a clinically relevant pharmacokinetic interaction for either compound.
Hydrochlorothiazide
A single oral dose of carvedilol 25 mg did not alter the pharmacokinetics of a single oral dose of hydrochlorothiazide 25 mg in 12 subjects with hypertension. Likewise, hydrochlorothiazide had no effect on the pharmacokinetics of carvedilol.
Rifampin
In a pharmacokinetic study conducted in 8 healthy male subjects, rifampin (600 mg daily for 12 days) decreased the AUC and Cmax of carvedilol by about 70% [see Drug Interactions (7.5)].
Torsemide
In a study of 12 healthy subjects, combined oral administration of carvedilol 25 mg once daily and torsemide 5 mg once daily for 5 days did not result in any significant differences in their pharmacokinetics compared with administration of the drugs alone.
Warfarin
Carvedilol (12.5 mg twice daily) did not have an effect on the steady-state prothrombin time ratios and did not alter the pharmacokinetics of R (+) - and S (-) -warfarin following concomitant administration with warfarin in 9 healthy volunteers.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
In 2-year studies conducted in rats given carvedilol at doses up to 75 mg/kg/day (12 times the MRHD as mg per m2) or in mice given up to 200 mg/kg/day (16 times the MRHD as mg per m2), carvedilol had no carcinogenic effect.
Carvedilol was negative when tested in a battery of genotoxicity assays, including the Ames and the CHO/ HGPRT assays for mutagenicity and the in vitro hamster micronucleus and in vivo human lymphocyte cell tests for clastogenicity.
In a combined fertility/developmental/post-natal toxicity study, rats were given carvedilol (12, 60, 300 mg per kg per day) orally by gavage for 2 weeks before mating and through mating, gestation, and weaning for females and for 62 days prior to and through mating for males. At a dosage of 300 mg per kg per day (greater than or equal to 50 times the MRHD as mg per m2) carvedilol was toxic to adult rats (sedation, reduced weight gain) and was associated with a reduced number of successful matings, prolonged mating time, fewer corpora lutea and implants per dam, fewer live pups per litter, and delays in physical growth/development. The no-effect level for overt toxicity and impairment of fertility was 60 mg per kg per day (10 times the MRHD as mg per m2).
-
14 CLINICAL STUDIES
14.1 Heart Failure
A total of 6,975 subjects with mild to severe heart failure were evaluated in placebo-controlled trials of carvedilol.
Mild-to-Moderate Heart Failure
Carvedilol was studied in 5 multicenter, placebo-controlled trials, and in 1 active-controlled trial (COMET trial) involving subjects with mild-to-moderate heart failure. Four US multicenter, double-blind, placebo-controlled trials enrolled 1,094 subjects (696 randomized to carvedilol) with NYHA class II-III heart failure and ejection fraction ≤0.35.
The vast majority were on digitalis, diuretics, and an ACE inhibitor at trial entry. Patients were assigned to the trials based upon exercise ability. An Australia-New Zealand double-blind, placebo-controlled trial enrolled 415 subjects (half randomized to carvedilol) with less severe heart failure. All protocols excluded subjects expected to undergo cardiac transplantation during the 7.5 to 15 months of double-blind follow-up. All randomized subjects had tolerated a 2-week course on carvedilol 6.25 mg twice daily.
In each trial, there was a primary end point, either progression of heart failure (1 US trial) or exercise tolerance (2 US trials meeting enrollment goals and the Australia-New Zealand trial).
There were many secondary end points specified in these trials, including NYHA classification, patient and physician global assessments, and cardiovascular hospitalization. Other analyses not prospectively planned included the sum of deaths and total cardiovascular hospitalizations. In situations where the primary end points of a trial do not show a significant benefit of treatment, assignment of significance values to the other results is complex, and such values need to be interpreted cautiously.
The results of the US and Australia-New Zealand trials were as follows:
Slowing Progression of Heart Failure
One US multicenter trial (366 subjects) had as its primary end point the sum of cardiovascular mortality, cardiovascular hospitalization, and sustained increase in heart failure medications. Heart failure progression was reduced, during an average follow-up of 7 months, by 48% (P = 0.008).
In the Australia-New Zealand trial, death and total hospitalizations were reduced by about 25% over 18 to 24 months. In the 3 largest US trials, death and total hospitalizations were reduced by 19%, 39%, and 49%, nominally statistically significant in the last 2 trials. The Australia-New Zealand results were statistically borderline.
Functional Measures
None of the multicenter trials had NYHA classification as a primary end point, but all such trials had it as a secondary end point. There was at least a trend toward improvement in NYHA class in all trials. Exercise tolerance was the primary end point in 3 trials; in none was a statistically significant effect found.
Subjective Measures
Health-related quality of life, as measured with a standard questionnaire (a primary end point in 1 trials), was unaffected by carvedilol. However, patients’ and investigators’ global assessments showed significant improvement in most trials.
Mortality
Death was not a pre-specified end point in any trial, but was analyzed in all trials. Overall, in these 4 US trials, mortality was reduced, nominally significantly so in 2 trials.
The COMET Trial
In this double-blind trial, 3,029 subjects with NYHA class II-IV heart failure (left ventricular ejection fraction ≤35%) were randomized to receive either carvedilol (target dose: 25 mg twice daily) or immediate-release metoprolol tartrate (target dose: 50 mg twice daily). The mean age of the subjects was approximately 62 years, 80% were males, and the mean left ventricular ejection fraction at baseline was 26%. Approximately 96% of the subjects had NYHA class II or III heart failure. Concomitant treatment included diuretics (99%), ACE inhibitors (91%), digitalis (59%), aldosterone antagonists (11%), and “statin” lipid-lowering agents (21%). The mean duration of follow-up was 4.8 years. The mean dose of carvedilol was 42 mg per day.
The trial had 2 primary end points: all-cause mortality and the composite of death plus hospitalization for any reason. The results of COMET are presented in Table 3 below. All-cause mortality carried most of the statistical weight and was the primary determinant of the trial size. All-cause mortality was 34% in the subjects treated with carvedilol and was 40% in the immediate-release metoprolol group (P = 0.0017; hazard ratio = 0.83, 95%CI: 0.74 to 0.93). The effect on mortality was primarily due to a reduction in cardiovascular death. The difference between the 2 groups with respect to the composite end point was not significant (P = 0.122). The estimated mean survival was 8.0 years with carvedilol and 6.6 years with immediate-release metoprolol.
Table 3. Results of COMET
End point
Carvedilol
N = 1,511
Metoprolol
N = 1,518
Hazard ratio
(95% CI)
All-cause mortality
34%
40%
0.83
0.74 – 0.93
Mortality + all hospitalization
74%
76%
0.94
0.86 – 1.02
Cardiovascular death
30%
35%
0.80
0.70 – 0.90
Sudden death
14%
17%
0.81
0.68 – 0.97
Death due to circulatory failure
11%
13%
0.83
0.67 – 1.02
Death due to stroke
0.9%
2.5%
0.33
0.18 – 0.62
It is not known whether this formulation of metoprolol at any dose or this low dose of metoprolol in any formulation has any effect on survival or hospitalization in patients with heart failure. Thus, this trial extends the time over which carvedilol manifests benefits on survival in 23 heart failure, but it is not evidence that carvedilol improves outcome over the formulation of metoprolol (TOPROL-XL®) with benefits in heart failure.
Severe Heart Failure (COPERNICUS):
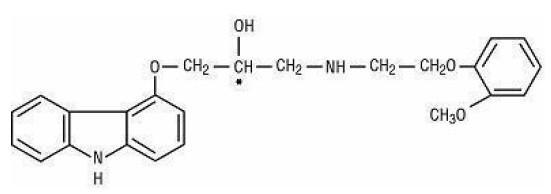
In a double-blind trial (COPERNICUS), 2,289 subjects with heart failure at rest or with minimal exertion and left ventricular ejection fraction <25% (mean 20%), despite digitalis (66%), diuretics (99%), and ACE inhibitors (89%) were randomized to placebo or carvedilol. Carvedilol was titrated from a starting dose of 3.125 mg twice daily to the maximum tolerated dose or up to 25 mg twice daily over a minimum of 6 weeks. Most subjects achieved the target dose of 25 mg. The trial was conducted in Eastern and Western Europe, the United States, Israel, and Canada. Similar numbers of subjects per group (about 100) withdrew during the titration period. The primary end point of the trial was all-cause mortality, but cause-specific mortality and the risk of death or hospitalization (total, cardiovascular [CV], or heart failure [HF]) were also examined. The developing trial data were followed by a data monitoring committee, and mortality analyses were adjusted for these multiple looks. The trial was stopped after a median follow-up of 10 months because of an observed 35% reduction in mortality (from 19.7% per patient-year on placebo to 12.8% on carvedilol, hazard ratio 0.65, 95% CI: 0.52 to 0.81,P = 0.0014, adjusted) (see Figure 1). The results of COPERNICUS are shown in Table 4.
Table 4. Results of COPERNICUS Trial in Subjects with Severe Heart Failure
End point
Placebo
(N = 1,133)
Carvedilol
(N = 1,156)
Hazard ratio
(95% Cl)
%
Reduction
Nominal
P value
Mortality
190
130
0.65
(0.52 –0.81)
35
0.00013
Mortality + all hospitalization
507
425
0.76
(0.67 – 0.87)
24
0.00004
Mortality + CV hospitalization
395
314
0.73
(0.63 – 0.84)
27
0.00002
Mortality + HF hospitalization
357
271
0.69
(0.59 – 0.81)
31
0.000004
Cardiovascular=CV; Heart failure=HF
Figure 1. Survival Analysis for COPERNICUS (Intent-to-Treat)
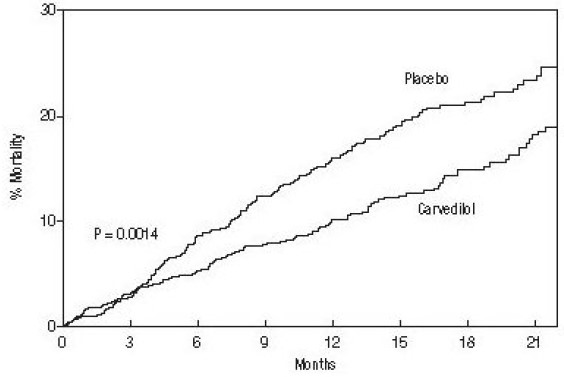
The effect on mortality was principally the result of a reduction in the rate of sudden death among subjects without worsening heart failure. Patients' global assessments, in which carvedilol-treated subjects were compared with placebo, were based on pre-specified, periodic patient self-assessments regarding whether clinical status post-treatment showed improvement, worsening or no change compared with baseline. Subjects treated with carvedilol showed significant improvements in global assessments compared with those treated with placebo in COPERNICUS. The protocol also specified that hospitalizations would be assessed. Fewer subjects on Carvedilol Tablets than on placebo were hospitalized for any reason (372 versus 432, P = 0.0029), for cardiovascular reasons (246 versus 314, P = 0.0003), or for worsening heart failure (198 versus 268, P = 0.0001). Carvedilol Tablets had a consistent and beneficial effect on all-cause mortality as well as the combined end points of all-cause mortality plus hospitalization (total, CV, or for heart failure) in the overall trial population and in all subgroups examined, including men and women, elderly and non-elderly, blacks and non-blacks, and diabetics and non-diabetics (see Figure 2).
Figure 2. Effects on Mortality for Subgroups in COPERNICUS
14.2 Left Ventricular Dysfunction Following Myocardial Infarction
CAPRICORN was a double-blind study comparing carvedilol and placebo in 1,959 subjects with a recent myocardial infarction (within 21 days) and left ventricular ejection fraction of less than or equal to 40%, with (47%) or without symptoms of heart failure. Subjects given carvedilol received 6.25 mg twice daily, titrated as tolerated to 25 mg twice daily. Subjects had to have a systolic blood pressure greater than 90 mm Hg, a sitting heart rate greater than 60 beats/minute, and no contraindication to β-blocker use. Treatment of the index infarction included aspirin (85%), IV or oral β-blockers (37%), nitrates (73%), heparin (64%), thrombolytics (40%), and acute angioplasty (12%). Background treatment included ACE inhibitors or angiotensin receptor blockers (97%), anticoagulants (20%), lipid-lowering agents (23%), and diuretics (34%). Baseline population characteristics included an average age of 63 years, 74% male, 95% Caucasian, mean blood pressure 121/74 mm Hg, 22% with diabetes, and 54% with a history of hypertension. Mean dosage achieved of carvedilol was 20 mg twice daily; mean duration of follow-up was 15 months.
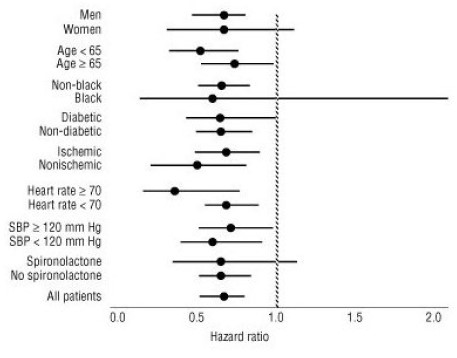
All-cause mortality was 15% in the placebo group and 12% in the carvedilol group, indicating a 23% risk reduction in patients treated with carvedilol (95% CI 2 to 40%, p = 0.03), as shown in Figure 3. The effects on mortality in various subgroups are shown in Figure 4. Nearly all deaths were cardiovascular (which were reduced by 25% by carvedilol), and most of these deaths were sudden or related to pump failure (both types of death were reduced by carvedilol). Another study end point, total mortality and all-cause hospitalization, did not show a significant improvement.
There was also a significant 40% reduction in fatal or non-fatal myocardial infarction observed in the group treated with carvedilol (95% CI 11% to 60%, p = 0.01). A similar reduction in the risk of myocardial infarction was also observed in a meta-analysis of placebo-controlled trials of carvedilol in heart failure.
Figure 3. Survival Analysis for CAPRICORN (Intent-to-Treat)
Figure 4. Effects on Mortality for Subgroups in CAPRICORN
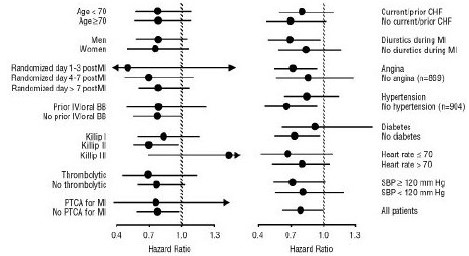
14.3 Hypertension
Carvedilol Tablet was studied in 2 placebo-controlled trials that utilized twice-daily dosing, at total daily doses of 12.5 to 50 mg. In these and other studies, the starting dose did not exceed 12.5 mg. At 50 mg/day, Carvedilol Tablet reduced sitting trough (12-hour) blood pressure by about 9/5.5 mm Hg; at 25 mg/day the effect was about 7.5/3.5 mm Hg. Comparisons of trough to peak blood pressure showed a trough to peak ratio for blood pressure response of about 65%. Heart rate fell by about 7.5 beats/minute at 50 mg/day. In general, as is true for other β-blockers, responses were smaller in black than non-black patients. There were no age- or gender-related differences in response.
The peak antihypertensive effect occurred 1 to 2 hours after a dose. The dose-related blood pressure response was accompanied by a dose-related increase in adverse effects [see Adverse Reactions (6)].
14.4 Hypertension With Type 2 Diabetes Mellitus
In a double-blind study (GEMINI), Carvedilol Tablet, added to an ACE inhibitor or angiotensin receptor blocker, was evaluated in a population with mild-to-moderate hypertension and well-controlled type 2 diabetes mellitus. The mean HbA1c at baseline was 7.2%. Carvedilol Tablet was titrated to a mean dose of 17.5 mg twice daily and maintained for 5 months. Carvedilol Tablet had no adverse effect on glycemic control, based on HbA1c measurements (mean change from baseline of 0.02%, 95% CI -0.06 to 0.10, p = NS) [see Warnings and Precautions (5.6)].
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Carvedilol Tablets are available as:
12.5 mg- White, oval shaped, film-coated tablet engraved with 256 on one side and plain on the other side, and contains 12.5 mg of Carvedilol.
- NDC: 71335-1813-1: 30 Tablets in a BOTTLE
- NDC: 71335-1813-2: 60 Tablets in a BOTTLE
- NDC: 71335-1813-3: 100 Tablets in a BOTTLE
- NDC: 71335-1813-4: 90 Tablets in a BOTTLE
- NDC: 71335-1813-5: 120 Tablets in a BOTTLE
- NDC: 71335-1813-6: 180 Tablets in a BOTTLE
- NDC: 71335-1813-7: 10 Tablets in a BOTTLE
Store at 25°C (77°F); Excursions permitted to 15°-30°C (59°-86°F) [see USP Controlled Room Temperature]. Protect from moisture. Dispense in a tight, light-resistant container as defined in the USP.
Repackaged/Relabeled by:
Bryant Ranch Prepack, Inc.
Burbank, CA 91504 -
17 PATIENT COUNSELING INFORMATION
See FDA-Approved Patient Labeling (Patient Information).
Advise the patient to read the FDA-approved patient labeling (Patient information).
Patients taking Carvedilol Tablet should be advised of the following:
Patients should take Carvedilol Tablet with food.
Patients should not interrupt or discontinue using Carvedilol Tablet without a physician's advice.
Patients with heart failure should consult their physician if they experience signs or symptoms of worsening heart failure such as weight gain or increasing shortness of breath.
Patients may experience a drop in blood pressure when standing, resulting in dizziness and, rarely, fainting. Patients should sit or lie down when these symptoms of lowered blood pressure occur.
If experiencing dizziness or fatigue, patients should avoid driving or hazardous tasks.
Patients should consult a physician if they experience dizziness or faintness, in case the dosage should be adjusted.
Diabetic patients should report any changes in blood sugar levels to their physician.
Contact lens wearers may experience decreased lacrimation.Manufactured by:
Beximco Pharmaceuticals Limited
126, Kathaldia, Tongi, Gazipur, 1711, Bangladesh
Mfg. Lic. No. 379 & 119
Manufactured for:
Beximco Pharmaceuticals USA Inc.
4110 Regal Oaks Drive, P.0. Box 1060, Suwanee, GA 30024, USA
Distributed by:
Bayshore Pharmaceuticals LLC
Short Hills, NJ 07078
3020009017
Revised - 07/2020 -
PATIENT PACKAGE INSERT
PATIENT INFORMATION – Rx only
Carvedilol Tablet
Read the Patient Information that comes with Carvedilol Tablet before you start taking them and each time you get a refill. There may be new information. This information does not take the place of talking with your doctor about your medical condition or your treatment. If you have any questions about Carvedilol Tablet, ask your doctor or pharmacist.
WHAT IS Carvedilol Tablet?
Carvedilol Tablet is a prescription medicine that belongs to a group of medicines called “betablockers”. Carvedilol Tablet is used, often with other medicines, for the following conditions:
- To treat patients with certain types of heart failure
- To treat patients with high blood pressure (hypertension)
- To treat patients who had a heart attack that worsened how well the heart pumps
Carvedilol Tablet is not approved for use in children under 18 years of age.
WHO SHOULD NOT TAKE CARVEDILOL Tablet?
Do not take Carvedilol Tablet if you:
- Have severe heart failure and are hospitalized in the intensive care unit or require certain intravenous medications that help support circulation (inotropic medications)
- Are prone to asthma or other breathing problems
- Have a slow heartbeat or a heart that skips a beat (irregular heartbeat)
- Have liver problems
- Are allergic to any of the ingredients in Carvedilol Tablet.
The active ingredient is Carvedilol Tablet. See the end of this leaflet for a list of all the ingredients in Carvedilol Tablet.
WHAT SHOULD I TELL MY DOCTOR BEFORE TAKING CARVEDILOL Tablet?
Tell your doctor about all of your medical conditions, including if you:
- Have asthma or other lung problems (such as bronchitis or emphysema)
- Have problems with blood flow in your feet and legs (peripheral vascular disease). Carvedilol Tablet can make some of your symptoms worse.
- Have diabetes
- Have thyroid problems
- Have a condition called pheochromocytoma
- Have had severe allergic reactions
- Are pregnant or trying to become pregnant. It is not known if Carvedilol Tablet is safe for your unborn baby. You and your doctor should talk about the best way to control your high blood pressure during pregnancy.
- Are breastfeeding. It is not known if Carvedilol Tablet passes into your breast milk. You should not breastfeed while using Carvedilol Tablet.
- Are scheduled for surgery and will be given anesthetic agents
- Are scheduled for cataract surgery and have taken or are currently taking Carvedilol Tablet.
- Are taking prescription or non-prescription medicines, vitamins, and herbal supplements. Carvedilol Tablet and certain other medicines can affect each other and cause serious side effects. Carvedilol Tablet may affect the way other medicines work. Also, other medicines may affect how well Carvedilol Tablet work.
Keep a list of all the medicines you take. Show this list to your doctor and pharmacist before you start a new medicine.
HOW SHOULD I TAKE Carvedilol Tablet?
It is important for you to take your medicine every day as directed by your doctor. If you stop taking Carvedilol Tablet suddenly, you could have chest pain and/or a heart attack. If your doctor decides that you should stop taking Carvedilol Tablet, your doctor may slowly lower your dose over a period of time before stopping it completely.
- Take Carvedilol Tablet exactly as prescribed. Your doctor will tell you how many Tablet to take and how often. In order to minimize possible side effects, your doctor might begin with a low dose and then slowly increase the dose.
- Do not stop taking Carvedilol Tablet and do not change the amount of Carvedilol Tablet you take without talking to your doctor.
- Tell your doctor if you gain weight or have trouble breathing while taking Carvedilol Tablet.
- Take Carvedilol Tablet with food.
- If you miss a dose of Carvedilol Tablet, take your dose as soon as you remember, unless it is time to take your next dose. Take your next dose at the usual time. Do not take 2 doses at the same time.
- If you take too many Carvedilol Tablet, call your doctor or poison control center right away.
WHAT SHOULD I AVOID WHILE TAKING Carvedilol Tablet?
- Carvedilol Tablet can cause you to feel dizzy, tired, or faint. Do not drive a car, use machinery, or do anything that needs you to be alert if you have these symptoms.
WHAT ARE POSSIBLE SIDE EFFECTS OF Carvedilol Tablet?
-
Low blood pressure (which may cause dizziness or fainting when you stand up). If these happen, sit or lie down right away and tell your doctor.
- Tiredness. If you feel tired or dizzy you should not drive, use machinery, or do anything that needs you to be alert.
- Slow heartbeat.
- Changes in your blood sugar. If you have diabetes, tell your doctor if you have any changes in your blood sugar levels.
- Carvedilol Tablet may hide some of the symptoms of low blood sugar, especially a fast heartbeat.
- Carvedilol Tablet may mask the symptoms of hyperthyroidism (overactive thyroid).
- Worsening of severe allergic reactions.
- Rare but serious allergic reactions (including hives or swelling of the face, lips, tongue, and/or throat that may cause difficulty in breathing or swallowing) have happened in patients who were on Carvedilol Tablet. These reactions can be life-threatening.
Other side effects of Carvedilol Tablet include shortness of breath, weight gain, diarrhea, and fewer tears or dry eyes that become bothersome if you wear contact lenses.
Call your doctor if you have any side effects that bother you or don't go away.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store Carvedilol Tablet?
- Store Carvedilol Tablet at less than 77°F (25°C). Keep the tablets dry.
- Safely, throw away Carvedilol Tablet that is out of date or no longer needed.
- Keep Carvedilol Tablet and all medicines out of the reach of children.
GENERAL INFORMATION ABOUT Carvedilol Tablet
Medicines are sometimes prescribed for conditions other than those described in patient information leaflets. Do not use Carvedilol Tablet for a condition for which they were not prescribed. Do not give Carvedilol Tablet to other people, even if they have the same symptoms you have. They may harm them.
This leaflet summarizes the most important information about Carvedilol Tablet. If you would like more information, talk with your doctor. You can ask your doctor or pharmacist for information about Carvedilol Tablet that is written for healthcare professionals
WHAT ARE THE INGREDIENTS IN Carvedilol Tablet?
Active Ingredient: Carvedilol
Inactive Ingredients: colloidal silicon dioxide, crospovidone, hypromellose, lactose monohydrate, magnesium stearate, microcrystalline cellulose, polyethylene glycol, sodium citrate dihydrate, sucrose, and titanium dioxide.
Carvedilol Tablet come in the following strengths: 3.125 mg, 6.25 mg, 12.5 mg and 25 mg
What is high blood pressure (hypertension)?
Blood pressure is the force of blood in your blood vessels when your heart beats and when your heart rests. You have high blood pressure when the force is too much. High blood pressure makes the heart work harder to pump blood through the body and causes damage to blood vessels. Carvedilol Tablets can help your blood vessels relax so your blood pressure is lower. Medicines that lower blood pressure may lower your chance of having a stroke or heart attack.
Manufactured by:
Beximco Pharmaceuticals Ltd.
126, Kathaldia, Tongi, Gazipur, 1711, Bangladesh.
Mfg. Lic. No. 379 & 119Manufactured for:
Beximco Pharmaceuticals USA Inc.
4110 Regal Oaks Drive, P.O. Box 1060, Suwanee, GA 30024, USA.Distributed by:
Bayshore Pharmaceuticals LLC
Short Hills, NJ 070783020009017
Revised - 07/2020
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CARVEDILOL
carvedilol tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 71335-1813(NDC:76385-112) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CARVEDILOL (UNII: 0K47UL67F2) (CARVEDILOL - UNII:0K47UL67F2) CARVEDILOL 12.5 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CROSPOVIDONE (UNII: 2S7830E561) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) SODIUM CITRATE, UNSPECIFIED FORM (UNII: 1Q73Q2JULR) SUCROSE (UNII: C151H8M554) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color white Score no score Shape OVAL (Biconvex) Size 12mm Flavor Imprint Code 256 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 71335-1813-1 30 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 07/17/2024 2 NDC: 71335-1813-2 60 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 06/11/2021 3 NDC: 71335-1813-3 100 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 09/06/2023 4 NDC: 71335-1813-4 90 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 08/10/2021 5 NDC: 71335-1813-5 120 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 07/17/2024 6 NDC: 71335-1813-6 180 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 10/27/2021 7 NDC: 71335-1813-7 10 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 07/17/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA078384 10/01/2016 Labeler - Bryant Ranch Prepack (171714327) Registrant - Bryant Ranch Prepack (171714327) Establishment Name Address ID/FEI Business Operations Bryant Ranch Prepack 171714327 REPACK(71335-1813) , RELABEL(71335-1813)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.
