RoyceDerm Sulfur Soap. by Shenzhen Situya Trading Co., Ltd. 82739-023 Complete
RoyceDerm Sulfur Soap. by
Drug Labeling and Warnings
RoyceDerm Sulfur Soap. by is a Otc medication manufactured, distributed, or labeled by Shenzhen Situya Trading Co., Ltd.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
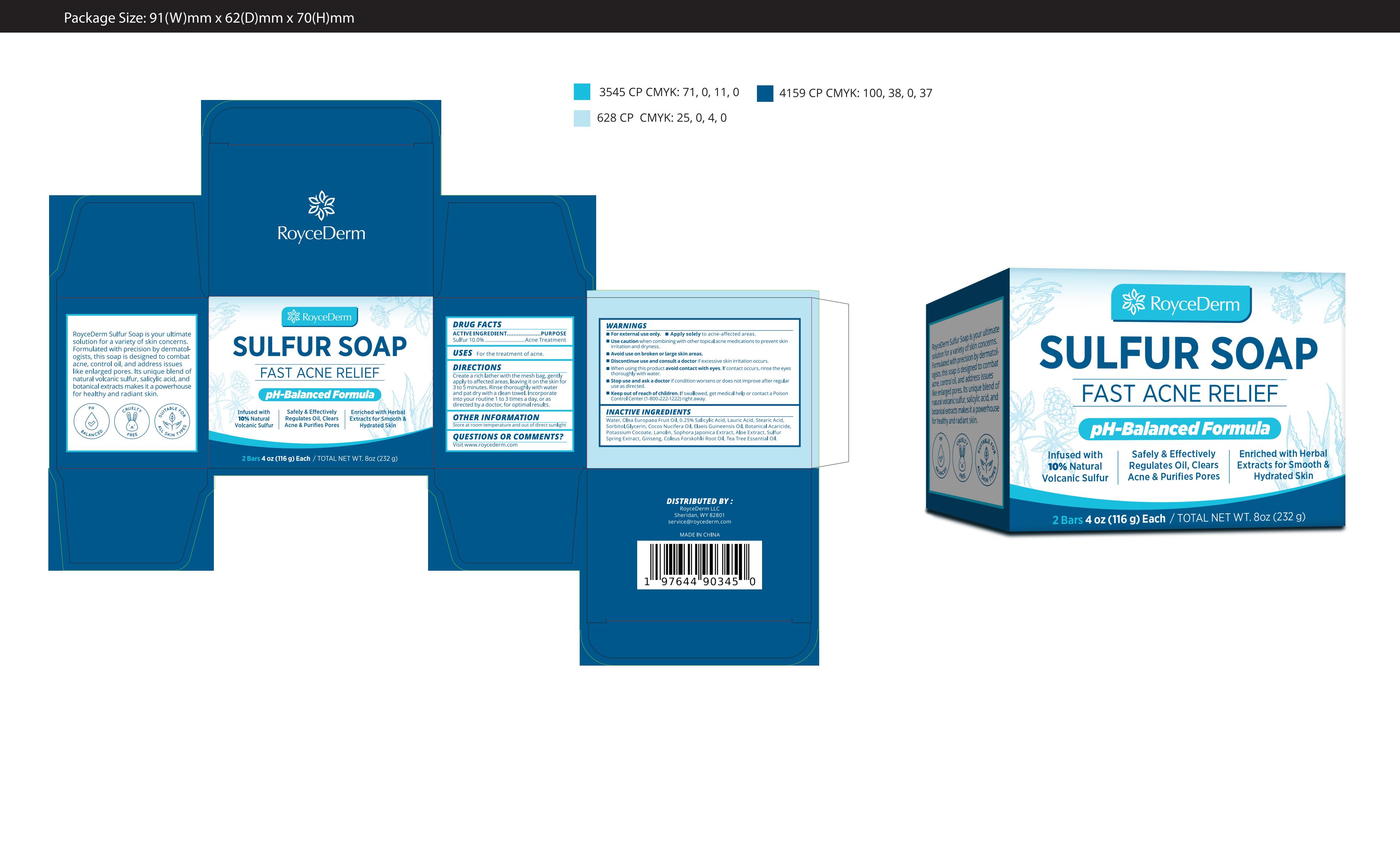
ROYCEDERM SULFUR SOAP.- sulfur soap soap
Shenzhen Situya Trading Co., Ltd.
----------
82739-023 Complete
Do not use
Use caution when combining with other topical acne medications to prevent skin irritation and dryness.
When Using
When using this product avoid contact with eyes.
If contact occurs, rinse the eyes thoroughly with water.
Stop Use
stop use and ask a doctor if condition worsens or does not improve after regular use as directed.
Keep Oot Of Reach Of Children
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center (1-800-222-1222) right away.
Directions
Create a rich lather with the mesh bag, gently apply to affected areas, leaving it on the skin for 3 to 5 minutes. Rinse thoroughly with water and pat dry with a clean towel. Incorporate into your routine 1 to 3 times a day, or as directed by a doctor, for optimal results.
Inactive ingredients
Water, Olea Europaea Fruit Oil, 0.25% Salicylic Acid, Lauric Acid, Stearic Acid, Sorbitol,Glycerin, Cocos Nucifera Oil, Elaeis Guineensis Oil, Botanical Acaricide, Potassium Cocoate, Lanolin, Sophora Japonica Extract, Aloe Extract, Sulfur Spring Extract, Ginseng, Coleus Forskohlii Root Oil, Tea Tree Essential Oil.
| ROYCEDERM SULFUR SOAP.
sulfur soap soap |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Labeler - Shenzhen Situya Trading Co., Ltd. (706154255) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Shenzhen Situya Trading Co., Ltd. | 706154255 | manufacture(82739-023) , label(82739-023) | |