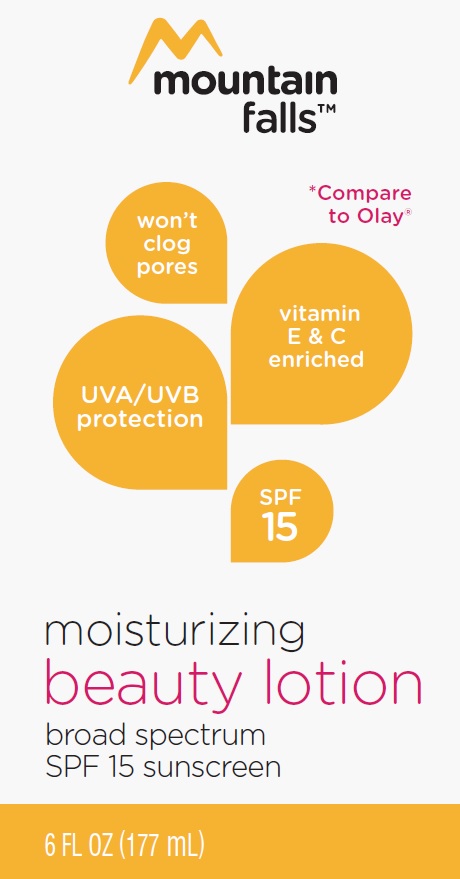
OCTINOXATE, ZINC OXIDE lotion
Octinoxate, Zinc Oxide by
Drug Labeling and Warnings
Octinoxate, Zinc Oxide by is a Otc medication manufactured, distributed, or labeled by Vi-Jon, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active ingredients
- Purpose
- Uses
- Warnings
- Do not use
- When using this product
- Stop use and ask a doctor if
- Keep out of reach of children.
-
Directions
- apply liberally 15 minutes before sun exposure
- reapply at least every 2 hours
- use a water resistant sunscreen if swimming or sweating
- Sun Protection Measures. spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk regularly use as sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- limit time in the sun, espcially from 10 a.m. - 2 p.m.
- wear long-sleeved shirts, pants, hats and sunglasses
- children under 6 months of age: ask a doctor
- Other information
-
inactive ingredients
water, glycerin, isohexadecane, sodium acrylate/sodium acryloyldimethyl taurate copolymer, polysorbate 80, laureth-7, cylopentasiloxane, cyclohexasiloxane, PEG/PPG-20/20 dimethicone, steareth-21, stearyl alcohol, Gossypium Herbaceum (cotton) seed oil, behenyl alcohol, cetyl alcohol, DMDM hydantoin, iodopropynyl butylcarbamate, fragrance, tocopheryl acetate, disodium EDTA, steareth-2, oleth-3 phosphate, ascorbic acid, panthenol
- disclaimer
- Adverse reactions
- adverse reaction
- principal display panel
-
INGREDIENTS AND APPEARANCE
OCTINOXATE, ZINC OXIDE
octinoxate, zinc oxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 0869-0909 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) Octinoxate 61.2 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (ZINC CATION - UNII:13S1S8SF37) ZINC CATION 30.6 mg in 1 mL Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) ISOHEXADECANE (UNII: 918X1OUF1E) SODIUM ACRYLATE/SODIUM ACRYLOYLDIMETHYLTAURATE COPOLYMER (4000000 MW) (UNII: 1DXE3F3OZX) POLYSORBATE 80 (UNII: 6OZP39ZG8H) LAURETH-7 (UNII: Z95S6G8201) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) CYCLOMETHICONE 6 (UNII: XHK3U310BA) PEG/PPG-20/20 DIMETHICONE (UNII: BA94B7CK8K) STEARETH-21 (UNII: 53J3F32P58) STEARYL ALCOHOL (UNII: 2KR89I4H1Y) LEVANT COTTONSEED OIL (UNII: N5CFT140R8) DOCOSANOL (UNII: 9G1OE216XY) CETYL ALCOHOL (UNII: 936JST6JCN) DMDM HYDANTOIN (UNII: BYR0546TOW) IODOPROPYNYL BUTYLCARBAMATE (UNII: 603P14DHEB) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) EDETATE DISODIUM (UNII: 7FLD91C86K) STEARETH-2 (UNII: V56DFE46J5) OLETH-3 PHOSPHATE (UNII: 8Q0Z18J1VL) ASCORBIC ACID (UNII: PQ6CK8PD0R) PANTHENOL (UNII: WV9CM0O67Z) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0869-0909-26 1 in 1 BOX 03/17/2016 1 118 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 2 NDC: 0869-0909-30 1 in 1 BOX 03/17/2016 2 177 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 03/17/2016 Labeler - Vi-Jon (790752542) Registrant - Vi-Jon (790752542) Establishment Name Address ID/FEI Business Operations Vi-Jon 790752542 manufacture(0869-0909)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.