HYDROCORTISONE BUTYRATE lotion
HYDROCORTISONE BUTYRATE by
Drug Labeling and Warnings
HYDROCORTISONE BUTYRATE by is a Prescription medication manufactured, distributed, or labeled by Mayne Pharma Inc., Teligent Pharma, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use HYDROCORTISONE BUTYRATE LOTION safely and effectively. See full prescribing information for HYDROCORTISONE BUTYRATE LOTION.
HYDROCORTISONE BUTYRATE Lotion 0.1%, for topical use.
Initial U.S. Approval: 1982INDICATIONS AND USAGE
Hydrocortisone Butyrate Lotion is a corticosteroid indicated for the topical treatment of mild to moderate atopic dermatitis in patients 3 months of age and older. (1)
DOSAGE AND ADMINISTRATION
- Apply a thin layer to the affected skin two times daily. (2)
- Rub in gently. (2)
- Discontinue Hydrocortisone Butyrate when control is achieved. (2)
- Reassess diagnosis if no improvement is seen within 2 weeks. Before prescribing for more than 2 weeks, any additional benefits of extending treatment to 4 weeks should be weighed against the risk of HPA axis suppression and local adverse events. Safety and efficacy of Hydrocortisone Butyrate Lotion has not been established beyond 4 weeks of use. (2)
- Avoid use under occlusion or in the diaper area. (2)
- Hydrocortisone Butyrate Lotion is not for oral, ophthalmic, or intravaginal use. (2)
DOSAGE FORMS AND STRENGTHS
Lotion, 0.1% (1 mg/g), supplied in bottles of 2 fl. oz. and 4 fl. oz. (3)
CONTRAINDICATIONS
None. (4)
WARNINGS AND PRECAUTIONS
- Reversible hypothalamic-pituitary-adrenal (HPA) axis suppression may occur, with the potential for glucocorticosteroid insufficiency. Consider periodic evaluation for HPA axis suppression if Hydrocortisone Butyrate Lotion is applied to large surface areas or used under occlusion. If HPA axis suppression is noted, reduce the application frequency, discontinue use, or switch to a lower potency corticosteroid. (5.1, 8.4)
- Systemic effects of topical corticosteroids may also include manifestations of Cushing's syndrome, hyperglycemia and glucosuria. (5.1, 8.4)
- Pediatric patients may be more susceptible to systemic toxicity due to their larger skin surface-to-body-mass ratios. (5.1, 8.4)
- Initiate appropriate therapy if concomitant skin infections develop. (5.2)
ADVERSE REACTIONS
The most common adverse re actions (> 1%) are application site re actions. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Teligent Pharma, Inc. at 1-856-697-1441 and/or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 6/2019
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hypothalamic-Pituitary-Adrenal (HPA) Axis Suppression
5.2 Concomitant Skin Infections
5.3 Allergic Contact Dermatitis
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
8 USE IN SPECIFIC POPULAT IONS
8.1 Pregnancy
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
Apply a thin layer to the affected skin areas two times daily, and rub in gently. Do not apply Hydrocortisone Butyrate Lotion in the diaper area unless directed by a physician.
Discontinue therapy when control is achieved. If no improvement is seen within 2 weeks, reassessment of the diagnosis may be necessary. Before prescribing for more than 2 weeks, any additional benefits of extending treatment to 4 weeks should be weighed against the risk of HPA axis suppression and local adverse events. The safety and efficacy of Hydrocortisone Butyrate Lotion has not been established beyond 4 weeks of use [see Warnings and Precautions (5.1)].
Do not use Hydrocortisone Butyrate Lotion with occlusive dressings unless directed by a physician. Avoid use in the diaper area, as diapers or plastic pants may constitute occlusive dressings.
Hydrocortisone Butyrate Lotion is not for oral, ophthalmic, or intravaginal use.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Hypothalamic-Pituitary-Adrenal (HPA) Axis Suppression
Systemic effects of topical corticosteroids may include reversible HPA axis suppression, manifestations of Cushing's syndrome, hyperglycemia, and glucosuria.
Studies conducted in pediatric subjects demonstrated reversible HPA axis suppression after use of Hydrocortisone Butyrate Lotion. Pediatric patients may be more susceptible than adults to systemic toxicity from equivalent doses of Hydrocortisone Butyrate Lotion due to their larger skin surface-to-body-mass ratios [see Use in Specific Populations (8.4)].
Patients applying a topical corticosteroid to a large surface area or to areas under occlusion should be considered for periodic evaluation of the HPA axis. This may be done by using cosyntropin (ACTH1- 24) stimulation testing (CST).
Minimize systemic corticosteroid effects by mitigating the risk factors for increased systemic absorption and using Hydrocortisone Butyrate Lotion as recommended [see Dosage and Administration (2)].
If HPA axis suppression is noted, the frequency of application should be reduced or the drug should be withdrawn, or a less potent corticosteroid should be substituted. Signs and symptoms of glucocorticosteroid insufficiency may occur, requiring supplemental systemic corticosteroids [see Adverse Reactions (6)].
5.2 Concomitant Skin Infections
If skin infections are present or develop, an appropriate antifungal, antibacterial or antiviral agent should be used. If a favorable response does not occur promptly, use of Hydrocortisone Butyrate Lotion should be discontinued until the infection has been adequately controlled [see Adverse Reactions (6)].
5.3 Allergic Contact Dermatitis
Allergic contact dermatitis with corticosteroids is usually diagnosed by observing a failure to heal rather than noticing a clinical exacerbation. Such an observation should be corroborated with appropriate patch testing. Discontinue Hydrocortisone Butyrate Lotion if the diagnosis is established [see Adverse Reactions (6)].
-
6 ADVERSE REACTIONS
The following adverse reactions are discussed in greater detail in other sections of the labeling:
- HPA axis suppression. This has been observed in pediatric subjects using Hydrocortisone Butyrate Lotion [see Warnings and Precautions (5.1) and Use in Specific Populations (8.4)]
- Concomitant skin infections [see Warnings and Precautions (5.2)]
- Allergic contact dermatitis [see Warnings and Precautions (5.3)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, the adverse reaction rates observed cannot be directly compared to rates in other clinical trials and may not reflect the rates observed in clinical practice.
The safety data derived from Hydrocortisone Butyrate Lotion clinical trials reflect exposure to Hydrocortisone Butyrate Lotion twice daily for up to four weeks in separate clinical trials involving pediatric subjects 3 months to 18 years of age and adult subjects 18 years of age and older with mild to moderate atopic dermatitis.
Adverse reactions shown in the tables below include those for which there is some basis to believe there is a causal relationship to Hydrocortisone Butyrate Lotion. Although the rates of application site reactions in the vehicle group were greater than those in the Hydrocortisone Butyrate group in both studies, these rates are included in the tables (Table 1 and Table 2) because skin irritation is a known adverse reaction of topical corticosteroids.
TABLE 1. Frequency of adverse reactions in pediatric subjects with mild to moderate atopic dermatitis Hydrocortisone Butyrate Lotion
(n=139) n (%)Vehicle
(n=145) n(%)Application site reactions, including application site burning, pruritus, dermatitis, erythema, eczema, inflammation, or irritation 2 (1) 20 (14) Infantile acne 1 (1) 0 (0) Skin depigmentation 1 (1) 0 (0) TABLE 2. Frequency of adverse reactions in adult subjects with mild to moderate atopic dermatitis Hydrocortisone Butyrate Lotion
(n=151) n (%)Vehicle
(n=150) n (%)Application site reactions, including application site burning, dermatitis, eczema, erythema, or pruritus 5 (3) 7 (5) 6.2 Postmarketing Experience
Because adverse reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
The following additional local adverse reactions have been reported infrequently with topical corticosteroids, and they may occur more frequently with the use of occlusive dressings and higher potency corticosteroids. These reactions included: irritation, folliculitis, acneiform eruptions, hypopigmentation, perioral dermatitis, allergic contact dermatitis, secondary infection, skin atrophy, striae, miliaria and telangiectasia.
-
8 USE IN SPECIFIC POPULAT IONS
8.1 Pregnancy
Pregnancy Category C.
There are no adequate and well-controlled studies in pregnant women. Therefore, Hydrocortisone Butyrate Lotion should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Corticosteroids have been shown to be teratogenic in laboratory animals when administered systemically at relatively low dosage levels. Some corticosteroids have been shown to be teratogenic after dermal application in laboratory animals.
Note: The animal multiples of human exposure calculations in this label were based on body surface area comparisons for an adult (i.e., mg/m2/day dose comparisons) assuming 100% human percutaneous absorption of a maximum topical human dose (MTHD) for hydrocortisone butyrate lotion (25 g lotion).
Systemic embryofetal development studies were conducted in rats and rabbits. Subcutaneous doses of 0.6, 1.8 and 5.4 mg/kg/day hydrocortisone butyrate were administered to pregnant female rats during gestation days 6 to17. In the presence of maternal toxicity, fetal effects noted at 5.4 mg/kg/day (2× MTHD) included an increased incidence of ossification variations and unossified sternebra. No treatment-related effects on embryofetal toxicity or teratogenicity were noted at doses of 5.4 mg/kg/day and 1.8 mg/kg/day, respectively (2× MTHD and 0.7× MTHD, respectively).
Subcutaneous doses of 0.1, 0.2 and 0.3 mg/kg/day hydrocortisone butyrate were administered to pregnant female rabbits during gestation days 7 to 20. An increased incidence of abortion was noted at 0.3 mg/kg/day (0.2× MTHD). In the absence of maternal toxicity, a dose-dependent decrease in fetal body weight was noted at doses ≥0.1 mg/kg/day (0.1× MTHD). Additional indicators of embryofetal toxicity (reduction in litter size, decreased number of viable fetuses, increased post-implantation loss) were noted at doses ≥0.2 mg/kg/day (0.2× MTHD). Additional fetal effects noted in this study included delayed ossification noted at doses ≥0.1 mg/kg/day and an increased incidence of fetal malformations (primarily skeletal malformations) noted at doses ≥0.2 mg/kg/day. A dose at which no treatment- related effects on embryofetal toxicity or teratogenicity were observed was not established in this study.
Additional systemic embryofetal development studies were conducted in rats and mice. Subcutaneous doses of 0.1 and 9 mg/kg/day hydrocortisone butyrate were administered to pregnant female rats during gestation days 9 to15. In the presence of maternal toxicity, an increase in fetal deaths and fetal resorptions and an increase in the number of ossifications in caudal vertebrae were noted at a dose of 9 mg/kg/day (3× MTHD). No treatment-related effects on embryofetal toxicity or teratogenicity were noted at 0.1 mg/kg/day (0.1× MTHD).
Subcutaneous doses of 0.2 and 1 mg/kg/day hydrocortisone butyrate were administered to pregnant female mice during gestation days 7 to 13. In the absence of maternal toxicity, an increased number of cervical ribs and one fetus with clubbed legs were noted at a dose of 1 mg/kg/day (0.2× MTHD). No treatment-related effects on embryofetal toxicity or teratogenicity were noted at doses of 1 and 0.2 mg/kg/day, respectively (0.2× MTHD and 0.1× MTHD, respectively).
No topical embryofetal development studies were conducted with hydrocortisone butyrate lotion. However, topical embryofetal development studies were conducted in rats and rabbits with a hydrocortisone butyrate ointment formulation. Topical doses of 1% and 10% hydrocortisone butyrate ointment were administered to pregnant female rats during gestation days 6 to15 or pregnant female rabbits during gestation days 6 to18. A dose-dependent increase in fetal resorptions was noted in rabbits (0.2 to 2× MTHD) and fetal resorptions were noted in rats at the 10% hydrocortisone butyrate ointment dose (80× MTHD). No treatment-related effects on embyrofetal toxicity were noted at the 1% hydrocortisone butyrate ointment dose in rats (8× MTHD). A dose at which no treatment-related effects on embryofetal toxicity were observed in rabbits after topical administration of hydrocortisone butyrate ointment was not established in this study. No treatment-related effects on teratogenicity were noted at a dose of 10% hydrocortisone butyrate ointment in rats or rabbits (80× MTHD and 2× MTHD, respectively).
A peri- and post-natal development study was conducted in rats. Subcutaneous doses of 0.6, 1.8 and 5.4 mg/kg/day hydrocortisone butyrate were administered to pregnant female rats from gestation day 6 to lactation day 20. In the presence of maternal toxicity, a dose-dependent decrease in fetal weight was noted at doses ≥ 1.8 mg/kg/day (0.7× MTHD). No treatment-related effects on fetal toxicity were noted at 0.6 mg/kg/day (0.2× MTHD). A delay in sexual maturation was noted at 5.4 mg/kg/day (2× MTHD). No treatment-related effects on sexual maturation were noted at 1.8 mg/kg/day. No treatment-related effects on behavioral development or subsequent reproductive performance were noted at 5.4 mg/kg/day.
8.3 Nursing Mothers
Systemically administered corticosteroids appear in human milk and could suppress growth, interfere with endogenous corticosteroid production, or cause other untoward effects. It is not known whether topical administration of corticosteroids could result in sufficient systemic absorption to produce detectable quantities in human milk. Because many drugs are excreted in human milk, caution should be exercised when Hydrocortisone Butyrate Lotion is administered to a nursing woman.
8.4 Pediatric Use
Safety and efficacy in pediatric patients below 3 months of age have not been established.
Because of higher skin surface-to-body-mass ratios, pediatric patients are at a greater risk than adults of HPA axis suppression when they are treated with topical corticosteroids [see Warnings and Precautions (5.1)]. They are therefore also at a greater risk of glucocorticosteroid insufficiency after withdrawal of treatment and of Cushing's syndrome while on treatment.
Eighty-four (84) pediatric subjects (3 months to less than 18 years of age) with moderate to severe atopic dermatitis affecting at least 25% of body surface area (BSA) treated with Hydrocortisone Butyrate Lotion three times daily for up to 4 weeks were assessed for HPA axis suppression. The disease severity (moderate to severe atopic dermatitis) and the dosing regimen (three times daily) in this HPA axis study were different from the subject population (mild to moderate atopic dermatitis) and the dosing regimen (two times daily) for which Hydrocortisone Butyrate Lotion is indicated. Seven of the 82 evaluable subjects (8.5%) demonstrated laboratory evidence of suppression, where the sole criterion for defining HPA axis suppression was a serum cortisol level of less than or equal to 18 micrograms per deciliter after cosyntropin stimulation. Suppressed subjects ranged in age from 1 to 12 years and, at the time of enrollment, had 35% to 90% BSA involvement. These subjects did not develop any other signs or symptoms of HPA axis suppression. At the first follow up visit, approximately one month after the conclusion of treatment, cosyntropin stimulation results of all subjects had returned to normal, with the exception of one subject. This last subject recovered adrenal function by the second post treatment visit, 55 days post-treatment.
Cushing's syndrome, linear growth retardation, delayed weight gain, and intracranial hypertension have also been reported in pediatric patients receiving topical corticosteroids. Manifestations of adrenal suppression in pediatric patients include low plasma cortisol levels to an absence of response to ACTH stimulation. Manifestations of intracranial hypertension include bulging fontanelles, headaches, and bilateral papilledema.
-
11 DESCRIPTION
Hydrocortisone Butyrate (hydrocortisone butyrate) Lotion, 0.1% contains hydrocortisone butyrate, a non-fluorinated hydrocortisone ester, for topical use. The chemical name of hydrocortisone butyrate is 11ß,17,21- Trihydroxypregn-4-ene-3,20-dione 17-butyrate. It has the following structural formula:
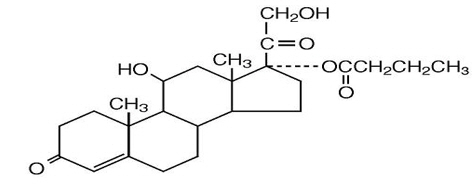
Hydrocortisone butyrate is a white to off white powder with a molecular weight of 432.56, and a molecular formula of C25H36O6. It is practically insoluble in water, slightly soluble in ether, soluble in methanol, in alcohol, and in acetone, and freely soluble in chloroform.
Each gram of Hydrocortisone Butyrate Lotion contains 1 mg of hydrocortisone butyrate in a white to off white lotion base consisting of anhydrous citric acid, ceteth-20, cetostearyl alcohol, butylated hydroxytoluene (BHT), butylparaben, light mineral oil, propylparaben, purified water, sodium citrate, and white petrolatum.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Topical corticosteroids share anti-inflammatory, antipruritic, and vasoconstrictive properties. The mechanism of the anti-inflammatory activity of the topical corticosteroids is unclear. However, corticosteroids are thought to act by the induction of phospholipase A2 inhibitory proteins, collectively called lipocortins. It is postulated that these proteins control the biosynthesis of potent mediators of inflammation such as prostaglandins and leukotrienes by inhibiting the release of their common precursor, arachidonic acid. Arachidonic acid is released from membrane phospholipids by phospholipase A2.
12.3 Pharmacokinetics
No studies were conducted to determine the pharmacokinetics of Hydrocortisone Butyrate Lotion.
The extent of percutaneous absorption of topical corticosteroids is determined by many factors, including the vehicle, the integrity of the epidermal barrier, and the use of occlusive dressings.
Topical corticosteroids can be absorbed through normal intact skin. Inflammation and/or other disease processes in the skin, occlusive dressings, or widespread application may increase percutaneous absorption.
Once absorbed through the skin, topical corticosteroids are handled through pharmacokinetic pathways similar to systemically administered corticosteroids.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
In a 2-year dermal rat carcinogenicity study with Hydrocortisone Butyrate Lotion, hydrocortisone butyrate was administered to Sprague-Dawley rats at topical doses of 0.05, 0.15, and 0.3 mg/kg/day in males and 0.1, 0.25, and 0.5 mg/kg/day in females (0.1% lotion). No drug-related tumors were noted in this study up to the highest doses evaluated in this study of 0.3 mg/kg/day in males (0.1× MTHD) and 0.5 mg/kg/day in females (0.2× MTHD).
Hydrocortisone butyrate revealed no evidence of mutagenic or clastogenic potential based on the results of two in vitro genotoxicity tests (Ames test and L5178Y/TK+/- mouse lymphoma assay) and one in vivo genotoxicity test (mouse micronucleus assay).
No evidence of impairment of fertility or effect on mating performance was observed in a fertility and general reproductive performance study conducted in male and female rats at subcutaneous doses up to and including 1.8 mg/kg/day (0.7× MTHD). Mild effects on maternal animals, such as reduced food consumption and a subsequent reduction in body weight gain, were seen at doses ≥ 0.6 mg/kg/day (0.2× MTHD).
-
14 CLINICAL STUDIES
In a multicenter, randomized, vehicle-controlled trial of 284 pediatric subjects 3 months to 18 years of age with mild to moderate atopic dermatitis, Hydrocortisone Butyrate Lotion or vehicle was applied twice daily for up to four weeks. Treatment success was assessed at day 29 (after 28 days of treatment) and was defined as the proportion of patients who achieved both "clear" or "almost clear" and at least a two grade improvement from baseline on a 5-point Physician's Global Assessment (PGA) scale. Study results are shown in Table 3.
TABLE 3. Efficacy results at Day 29 in pediatric subjects Hydrocortisone Butyrate Lotion (n=139) Vehicle (n=145) Number (%) successes 68 (49%) 35 (24%) Another multicenter, randomized, double-blind study compared twice daily treatment with Hydrocortisone Butyrate Lotion (n=151) to vehicle (n=150) for three or four weeks in adult subjects (ages 18 years or older), having atopic dermatitis, including those with mild or moderate severity. Results favored Hydrocortisone Butyrate Lotion over vehicle.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Hydrocortisone Butyrate Lotion, 0.1%, is white to off white in color and supplied in bottles of 2 fl. oz. (NDC: 51862-159-02) and 4 fl. oz. (NDC: 51862-159-04).
-
17 PATIENT COUNSELING INFORMATION
Patients using Hydrocortisone Butyrate Lotion should receive the following information and instructions:
- Apply a thin layer to the affected skin two times daily, and rub in gently.
- Discontinue Hydrocortisone Butyrate Lotion when control is achieved.
- Do not use for longer than 4 weeks.
- Avoid contact with the eyes.
- Do not bandage, otherwise cover, or wrap the affected skin area so as to be occlusive unless directed by physician.
- Do not use Hydrocortisone Butyrate Lotion in the diaper area, as diapers or plastic pants may constitute occlusive dressings.
- Do not use Hydrocortisone Butyrate Lotion on the face, underarms, or groin areas unless directed by physician.
- If no improvement is seen within 2 weeks, contact physician.
- Do not use other corticosteroid-containing products while using Hydrocortisone Butyrate Lotion without first consulting physician.
- SPL UNCLASSIFIED SECTION
-
PRINCIPAL DISPLAY PANEL - 118 mL Bottle Carton
NDC: 51862-159-04
Hydrocortisone Butyrate
Lotion0.1%
For topical use only.
Not for eye use.Rx Only
4 fl oz
(118 mL)mayne pharma
-
INGREDIENTS AND APPEARANCE
HYDROCORTISONE BUTYRATE
hydrocortisone butyrate lotionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 51862-159 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HYDROCORTISONE BUTYRATE (UNII: 05RMF7YPWN) (HYDROCORTISONE - UNII:WI4X0X7BPJ) HYDROCORTISONE BUTYRATE 1 mg in 1 mL Inactive Ingredients Ingredient Name Strength ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) CETETH-20 (UNII: I835H2IHHX) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) BUTYLPARABEN (UNII: 3QPI1U3FV8) LIGHT MINERAL OIL (UNII: N6K5787QVP) PROPYLPARABEN (UNII: Z8IX2SC1OH) WATER (UNII: 059QF0KO0R) SODIUM CITRATE, UNSPECIFIED FORM (UNII: 1Q73Q2JULR) PETROLATUM (UNII: 4T6H12BN9U) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 51862-159-04 1 in 1 CARTON 11/29/2019 1 118 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA209556 11/29/2019 Labeler - Mayne Pharma Inc. (867220261) Establishment Name Address ID/FEI Business Operations Teligent Pharma, Inc. 011036910 MANUFACTURE(51862-159)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.