RHEUMATOID ARTHRITIS PAIN MANAGEMENT- horse chestnut, caulophyllum thalictroides root, phytolacca americana root, ruta graveolens flowering top, and silicon dioxide solution/ drops
Rheumatoid Arthritis Pain Management by
Drug Labeling and Warnings
Rheumatoid Arthritis Pain Management by is a Homeopathic medication manufactured, distributed, or labeled by Forces of Nature. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
-
ACTIVE INGREDIENT
Active Ingredient Purpose - * Certified Organic
Aesculus Hippocastanum* 12X, Relieves Soreness Caulophyllum Thalictroides* 3X Relief from Joint Pain and Stiffness in Joints Ruta Graveolens* 30C Relief from Sore Aching Joints Silica* 12X Relief from Joint Pain Associated with Rheumatism Phytollacca Americana* 12C Relief from Rheumatic Stiffness - Also Contains
- Indications
- Directions
-
Warnings
For external use only. Avoid contact with the eyes; if contact occurs, flush with water and seek advice from medical personnel.
If condition worsens or does not improve after regular use of product as directed, consult a doctor. Do not apply to wounds or damaged skin, use for prolonged periods, or use over raw surfaces or blistered areas without consulting a doctor.
- SPL UNCLASSIFIED SECTION
-
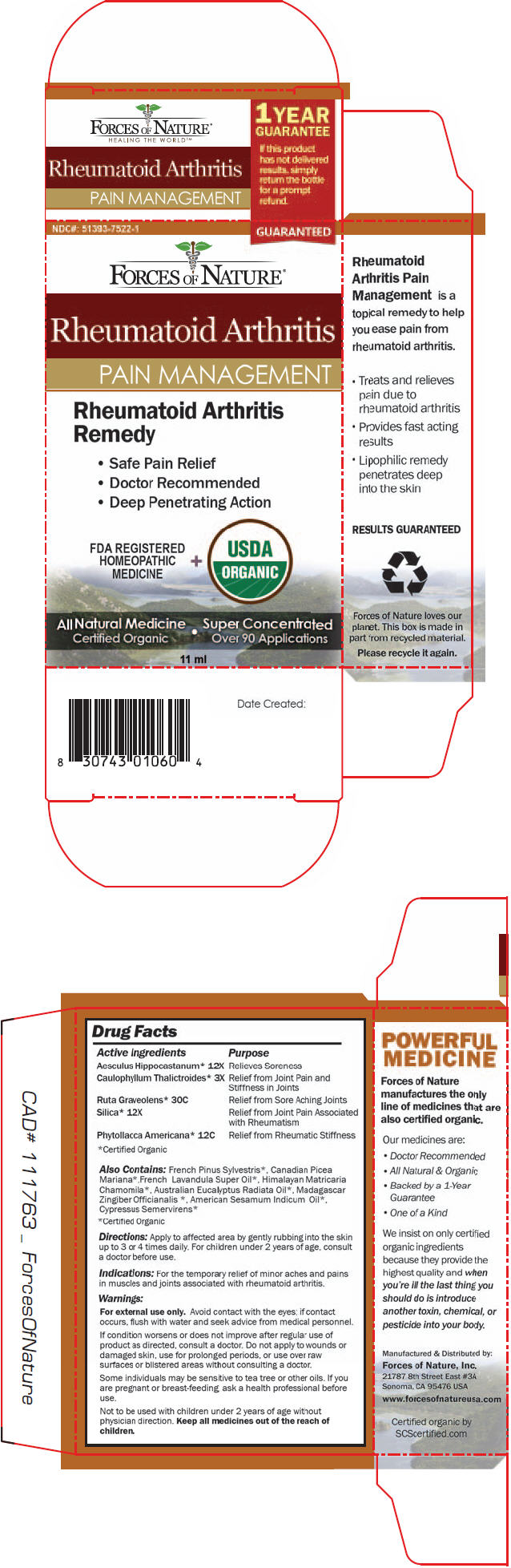
PRINCIPAL DISPLAY PANEL - 11 ml Bottle Box
NDC#: 51393-7522-1
GUARANTEEDFORCES OF NATURE®
Rheumatoid Arthritis
PAIN MANAGEMENT
Rheumatoid Arthritis
Remedy- Safe Pain Relief
- Doctor Recommended
- Deep Penetrating Action
FDA REGISTERED
HOMEOPATHIC
MEDICINE
+
USDA
ORGANICAll Natural Medicine
Certified Organic
Super Concentrated
Over 90 Applications11 ml
-
INGREDIENTS AND APPEARANCE
RHEUMATOID ARTHRITIS PAIN MANAGEMENT
horse chestnut, caulophyllum thalictroides root, phytolacca americana root, ruta graveolens flowering top, and silicon dioxide solution/ dropsProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 51393-7522 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Horse Chestnut (UNII: 3C18L6RJAZ) (Horse Chestnut - UNII:3C18L6RJAZ) Horse Chestnut 12 [hp_C] in 100 mL Caulophyllum Thalictroides Root (UNII: JTJ6HH6YEH) (Caulophyllum Thalictroides Root - UNII:JTJ6HH6YEH) Caulophyllum Thalictroides Root 3 [hp_X] in 100 mL Phytolacca Americana Root (UNII: 11E6VI8VEG) (Phytolacca Americana Root - UNII:11E6VI8VEG) Phytolacca Americana Root 12 [hp_C] in 100 mL Ruta Graveolens Flowering Top (UNII: N94C2U587S) (Ruta Graveolens Flowering Top - UNII:N94C2U587S) Ruta Graveolens Flowering Top 30 [hp_C] in 100 mL Silicon Dioxide (UNII: ETJ7Z6XBU4) (Silicon Dioxide - UNII:ETJ7Z6XBU4) Silicon Dioxide 12 [hp_X] in 100 mL Inactive Ingredients Ingredient Name Strength Pine Needle Oil (PINUS SYLVESTRIS) (UNII: 5EXL5H740Y) Picea Mariana Leaf Oil (UNII: Q1J49L1A5O) Lavandin Oil (UNII: 9RES347CKG) Matricaria Chamomilla Flowering Top Oil (UNII: SA8AR2W4ER) Eucalyptus Radiata Leaf Oil (UNII: SB9A7612BD) Ginger (UNII: C5529G5JPQ) Sesame Oil (UNII: QX10HYY4QV) Cupressus Sempervirens Whole (UNII: V0IW9P606J) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 51393-7522-1 1 in 1 BOX 1 11 mL in 1 BOTTLE, DISPENSING 2 NDC: 51393-7522-2 1 in 1 BOX 2 33 mL in 1 BOTTLE, DISPENSING Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date UNAPPROVED HOMEOPATHIC 01/01/2013 Labeler - Forces of Nature (050169130)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.