aramarkNeutral-Eyes
Neutral-Eyes by
Drug Labeling and Warnings
Neutral-Eyes by is a Otc medication manufactured, distributed, or labeled by Western First Aid Safety. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
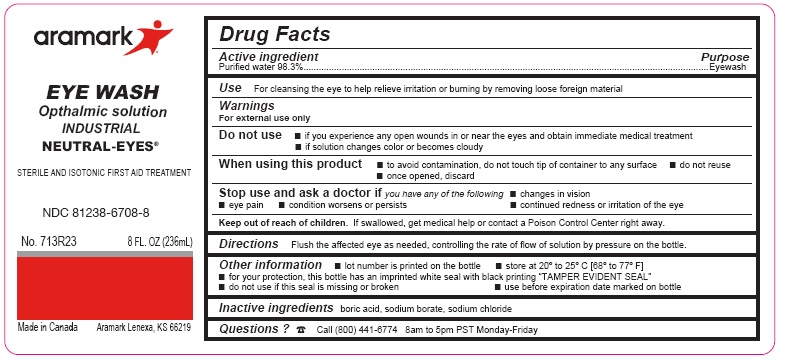
NEUTRAL-EYES- purified water 98.3% solution
Western First Aid Safety
----------
aramarkNeutral-Eyes
Warnings
For external use only
Do not use■ if you experience any open wounds in or near the eyes
and get medical help right away
■ if solution changes color or becomes cloudy
When using this product ■ to avoid
contamination, do not touch tip of container to any
surface ■ do not reuse ■ once opened, discard
Stop use and ask a doctor ifyou experience
■ eye pain ■ changes in vision ■ continued redness
or irritation of the eye ■ condition worsens or persists
Keep out of reach of children.If swallowed, get
medical help or contact a Poison Control Center
right away.
DirectionsFlush the affected eye as needed,
controlling the rate of flow of solution by pressure on
the bottle
| NEUTRAL-EYES
purified water 98.3% solution |
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
| Labeler - Western First Aid Safety (043861524) |
| Registrant - Western First Aid Safety (043861524) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Niagara Pharmaceuticals, inc. | 205477792 | manufacture(81238-6708) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| STERIS Applied Sterilization Technologies ULC | 243378155 | sterilize(81238-6708) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Isomedix Operations Inc. | 093889061 | sterilize(81238-6708) | |
Trademark Results [Neutral-Eyes]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 NEUTRAL-EYES 73429482 1283010 Dead/Cancelled |
AFASSCO, Inc. 1983-06-09 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.