84167-003 Completed
UROSE Full Coverage Cream by
Drug Labeling and Warnings
UROSE Full Coverage Cream by is a Otc medication manufactured, distributed, or labeled by Guangzhou Meixi Biotechnology Co., Ltd.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
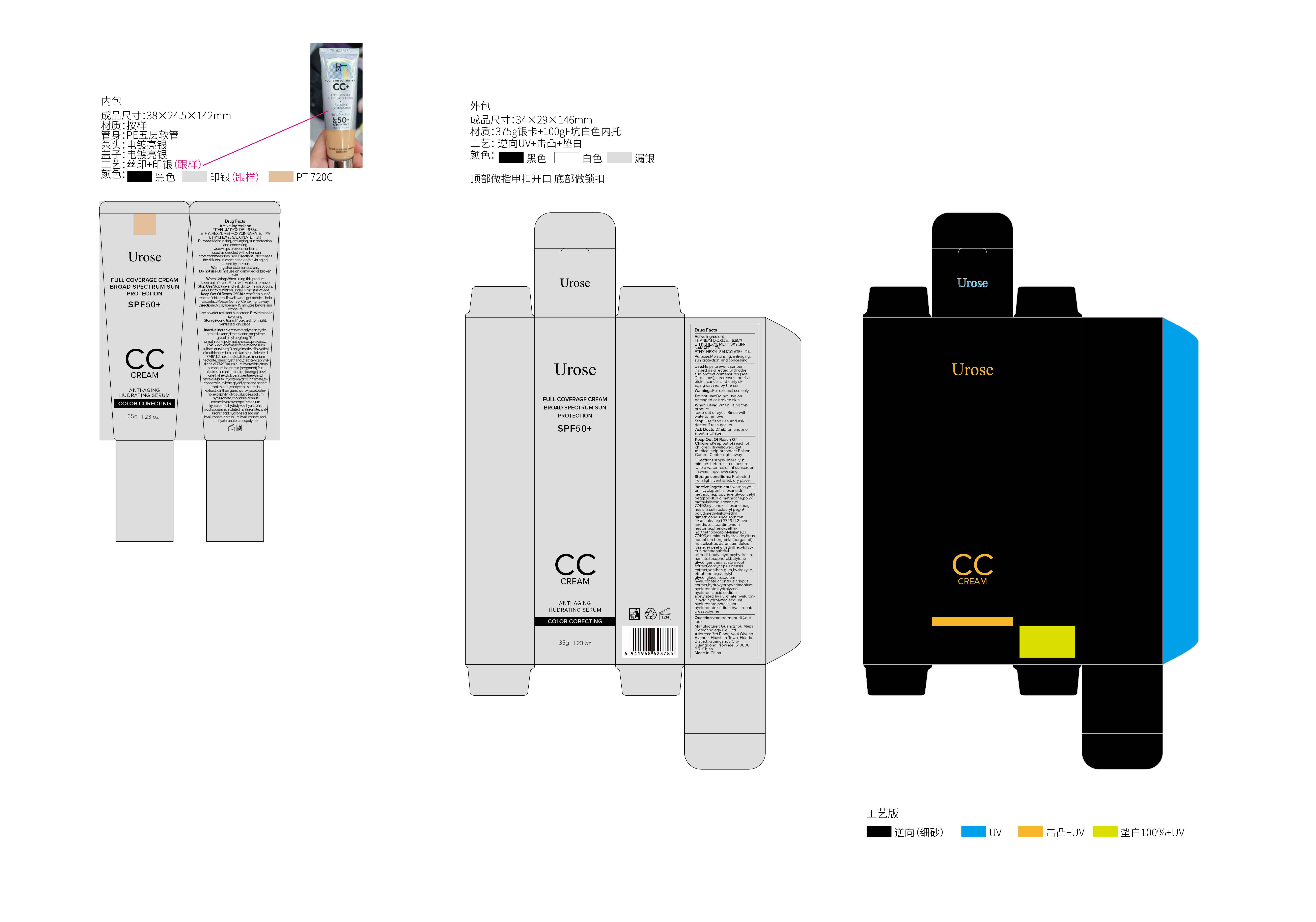
UROSE FULL COVERAGE CREAM- full coverage cream cream
Guangzhou Meixi Biotechnology Co., Ltd.
----------
84167-003 Completed
Use
Product features: touch the skin as smooth as cream mousse, and facial integration to make the entire makeup naturally flawless, more texture, just the right amount of gloss, as if born with flawless skin, to create the exclusive elegant fog makeup feeling.
When Using
When using this product
keep out of eyes. Rinse with wate to remove
Stop use and ask doctor if rash occurs.
Keep Oot Of Reach Of Children
Keep out of reach of children. lfswallowed, get medical help orcontact Poison Control Center right away
Directions
After basic skin care, apply liberally and evenly 15 minutes prior to sun exposure. Or gently dot on the forehead, cheeks, chin and nose wings, and then spread evenly on the skin surface
Inactive ingredients
WATER,GLYCERIN,CYCLOPENTASILOXANE,DIMETHICONE,PROPYLENE GLYCOL,CETYL PEG/PPG-10/1 DIMETHICONE,POLYMETHYLSILSESQUIOXANE,CI 77492,CYCLOHEXASILOXANE,MAGNESIUM SULFATE,LAURYL PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE,SILICA,SORBITAN SESQUIOLEATE,CI 77491,1,2-HEXANEDIOL,DISTEARDIMONIUM HECTORITE,PHENOXYETHANOL,TRIETHOXYCAPRYLYLSILANE,CI 77499,ALUMINUM HYDROXIDE,CITRUS AURANTIUM BERGAMIA (BERGAMOT) FRUIT OIL,CITRUS AURANTIUM DULCIS (ORANGE) PEEL OIL,ETHYLHEXYLGLYCERIN,PENTAERYTHRITYL TETRA-DI-T-BUTYL HYDROXYHYDROCINNAMATE,TOCOPHEROL,BUTYLENE GLYCOL,GENTIANA SCABRA ROOT EXTRACT,CORDYCEPS SINENSIS EXTRACT,XANTHAN GUM,HYDROXYACETOPHENONE,CAPRYLYL GLYCOL,GLUCOSE,SODIUM HYALURONATE,CHONDRUS CRISPUS EXTRACT,HYDROXYPROPYLTRIMONIUM HYALURONATE,HYDROLYZED HYALURONIC ACID,SODIUM ACETYLATED HYALURONATE,HYALURONIC ACID,HYDROLYZED SODIUM HYALURONATE,POTASSIUM HYALURONATE,SODIUM HYALURONATE CROSSPOLYMER
| UROSE FULL COVERAGE CREAM
full coverage cream cream |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Guangzhou Meixi Biotechnology Co., Ltd. (625724571) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Guangzhou Meixi Biotechnology Co., Ltd. | 625724571 | manufacture(84167-003) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.