These highlights do not include all the information needed to use FENOFIBRATE. See full prescribing information for FENOFIBRATE and Initial U.S. Approval
FENOFIBRATE by
Drug Labeling and Warnings
FENOFIBRATE by is a Prescription medication manufactured, distributed, or labeled by Basiem. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
FENOFIBRATE- fenofibrate tablet
Basiem
----------
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use FENOFIBRATE. See full prescribing information for FENOFIBRATE and Initial U.S. Approval
RECENT MAJOR CHANGESWarnings and Precautions (5.9) INDICATIONS AND USAGEFENOFIBRATE is a peroxisome proliferator receptor-activated receptor (PPAR) alpha agonist indicated as an adjunct to diet: (1) to reduce elevated LDL-C, Total-C, TG, and Apo B, and to increase HDL-C in adult patients with primary hypercholesterolemia or mixed dyslipidemia. (1.1) Important Limitations of Use: Fenofibrate was not shown to reduce coronary heart disease morbidity and mortality in patients with type 2 diabetes mellitus. (5.1) (1) DOSAGE FORMS AND STRENGTHSOral tablets: 160 mg (3) CONTRAINDICATIONSWARNINGS AND PRECAUTIONS
ADVERSE REACTIONSDRUG INTERACTIONSSee 17 for PATIENT COUNSELING INFORMATION. Revised: 3/2019 |
FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
1.1 Primary Hypercholesterolemia or Mixed Dyslipidemia
FENOFIBRATE is indicated as adjunctive therapy to diet to reduce elevated low-density lipoprotein cholesterol (LDL-C), total cholesterol (Total-C), Triglycerides, and apolipoprotein B (Apo B), and to increase high-density lipoprotein cholesterol (HDL-C) in adult patients with primary hypercholesterolemia or mixed dyslipidemia.
1.2 Severe Hypertriglyceridemia
FENOFIBRATE is also indicated as adjunctive therapy to diet for treatment of adult patients with severe hypertriglyceridemia. Improving glycemic control in diabetic patients showing fasting chylomicronemia will usually reduce fasting triglycerides and eliminate chylomicronemia thereby obviating the need for pharmacologic intervention.
Markedly elevated levels of serum triglycerides (e.g., >2,000 mg/dL) may increase the risk of developing pancreatitis. The effect of fenofibrate therapy on reducing this risk has not been adequately studied.
1.3 Important Limitations of Use
Fenofibrate was not shown to reduce coronary heart disease morbidity and mortality in a large, randomized controlled trial of patients with type 2 diabetes mellitus [see Warnings and Precautions (5.1)].
2 DOSAGE AND ADMINISTRATION
The dose of FENOFIBRATE is 160 mg once daily.
Patients should be placed on an appropriate lipid-lowering diet before receiving FENOFIBRATE and should continue this diet during treatment with FENOFIBRATE.
Lipid levels should be monitored periodically. Therapy should be withdrawn in patients who do not have an adequate response after two months of treatment.
FENOFIBRATE tablets can be given without regard to meals. Patients should be advised to swallow Triglide tablets whole. Do not crush, break, dissolve, or chew tablets.
4 CONTRAINDICATIONS
FENOFIBRATE is contraindicated in:
- Patients with severe renal impairment, including those with end-stage renal disease (ESRD) and those receiving dialysis [see Clinical Pharmacology (12.3)].
- Patients with active liver disease, including those with primary biliary cirrhosis and unexplained persistent liver function abnormalities [see Warning and Precautions (5.3)].
- Patients with preexisting gallbladder disease [see Warning and Precautions (5.5)].
- Patients who have a known hypersensitivity to fenofibrate or fenofibric acid [see Warning and Precautions (5.9)].
- Nursing mothers [see Use in Specific Populations (8.3)].
5 WARNINGS AND PRECAUTIONS
5.1 Mortality and Coronary Heart Disease Morbidity
The effect of FENOFIBRATE on coronary heart disease morbidity and mortality and non-cardiovascular mortality has not been established.
The Action to Control Cardiovascular Risk in Diabetes Lipid (ACCORD Lipid) trial was a randomized placebo-controlled study of 5518 patients with type 2 diabetes mellitus on background statin therapy treated with fenofibrate. The mean duration of follow-up was 4.7 years. Fenofibrate plus statin combination therapy showed a non-significant 8% relative risk reduction in the primary outcome of major adverse cardiovascular events (MACE), a composite of non-fatal myocardial infarction, non-fatal stroke, and cardiovascular disease death (hazard ratio [HR] 0.92, 95% CI 0.79-1.08) (p=0.32) as compared to statin monotherapy. In a gender subgroup analysis, the hazard ratio for MACE in men receiving combination therapy versus statin monotherapy was 0.82 (95% CI 0.69-0.99), and the hazard ratio for MACE in women receiving combination therapy versus statin monotherapy was 1.38 (95% CI 0.98-1.94) (interaction p=0.01). The clinical significance of this subgroup finding is unclear.
The Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study was a 5-year randomized, placebo-controlled study of 9,795 patients with type 2 diabetes mellitus treated with fenofibrate. Fenofibrate demonstrated a non-significant 11% relative reduction in the primary outcome of coronary heart disease events (hazard ratio [HR] 0.89, 95% CI 0.75-1.05, p=0.16) and a significant 11% reduction in the secondary outcome of total cardiovascular disease events (HR 0.89 [0.80-0.99], p=0.04). There was a non-significant 11% (HR 1.11 [0.95, 1.29], p=0.18) and 19% (HR 1.19 [0.90, 1.57], p=0.22) increase in total and coronary heart disease mortality, respectively, with fenofibrate as compared to placebo.
Because of chemical, pharmacological, and clinical similarities between FENOFIBRATE, clofibrate, and gemfibrozil, the adverse findings in 4 large randomized, placebo-controlled clinical studies with these other fibrate drugs may also apply to Triglide.
In the Coronary Drug Project, a large study of post myocardial infarction of patients treated for 5 years with clofibrate, there was no difference in mortality seen between the clofibrate group and the placebo group. There was however, a difference in the rate of cholelithiasis and cholecystitis requiring surgery between the two groups (3.0% vs. 1.8%).
In a study conducted by the World Health Organization (WHO), 5000 subjects without known coronary artery disease were treated with placebo or clofibrate for 5 years and followed for an additional one year. There was a statistically significant, higher age-adjusted all-cause mortality in the clofibrate group compared with the placebo group (5.70% vs. 3.96%, p=<0.01). Excess mortality was due to a 33% increase in non-cardiovascular causes, including malignancy, post-cholecystectomy complications, and pancreatitis. This appeared to confirm the higher risk of gallbladder disease seen in clofibrate-treated patients studied in the Coronary Drug Project.
The Helsinki Heart Study was a large (n=4,081) study of middle-aged men without a history of coronary artery disease. Subjects received either placebo or gemfibrozil for 5 years, with a 3.5 year open extension afterward. Total mortality was numerically higher in the gemfibrozil randomization group but did not achieve statistical significance (p=0.19, 95% confidence interval for relative risk G:P=0.91-1.64). Although cancer deaths trended higher in the gemfibrozil group (p=0.11), cancers (excluding basal cell carcinoma) were diagnosed with equal frequency in both study groups. Due to the limited size of the study, the relative risk of death from any cause was not shown to be different than that seen in the 9 year follow-up data from the WHO study (RR=1.29).
A secondary prevention component of the Helsinki Heart Study enrolled middle-aged men excluded from the primary prevention study because of known or suspected coronary heart disease. Subjects received gemfibrozil or placebo for 5 years. Although cardiac deaths trended higher in the gemfibrozil group, this was not statistically significant (hazard ratio 2.2, 95% confidence interval: 0.94-5.05).
5.2 Skeletal Muscle
Fenofibrates increase the risk of myopathy and have been associated with rhabdomyolysis. The risk for serious muscle toxicity appears to be increased in elderly patients and in patients with diabetes, renal insufficiency, or hypothyroidism.
Data from observational studies indicate that the risk for rhabdomyolysis is increased when fibrates, in particular gemfibrozil, are co-administered with an HMG-CoA reductase inhibitor (statin). The combination should be avoided unless the benefit of further alterations in lipid levels is likely to outweigh the increased risk of this drug combination [see Clinical Pharmacology (12.3)].
Myopathy should be considered in any patient with diffuse myalgias, muscle tenderness or weakness, and/or marked elevations of creatine phosphokinase (CPK) levels.
Patients should be advised to report promptly unexplained muscle pain, tenderness or weakness, particularly if accompanied by malaise or fever. CPK levels should be assessed in patients reporting these symptoms, and Triglide therapy should be discontinued if markedly elevated CPK levels occur or myopathy/myositis is suspected.
Cases of myopathy, including rhabdomyolysis, have been reported with fenofibrates coadministered with colchicine, and caution should be exercised when prescribing fenofibrate with colchicine [see Drug Interactions (7.4)].
5.3 Liver Function
Fenofibrate can increase serum transaminases [AST (SGOT) or ALT (SGPT)].
In a pooled analysis of 10 placebo-controlled trials, increases to >3 times the upper limit of normal occurred in 5.3% of patients taking fenofibrate versus 1.1% of patients treated with placebo. When transaminase determinations were followed either after discontinuation of treatment or during continued treatment, a return to normal limits was usually observed. The incidence of increases in transaminases related to fenofibrate therapy appear to be dose related. In an 8-week dose-ranging study, the incidence of ALT or AST elevations to at least three times the upper limit of normal was 13% in patients receiving dosages equivalent to 134 mg to 200 mg fenofibrate per day (the high dose equivalent to 160 mg Triglide) and was 0% in those receiving dosages equivalent to 34 mg or 67 mg micronized fenofibrate per day, or placebo.
Hepatocellular, chronic active and cholestatic hepatitis associated with fenofibrate therapy have been reported after exposures of weeks to several years. In extremely rare cases, cirrhosis has been reported in association with chronic active hepatitis.
Baseline and regular periodic monitoring of liver tests, including serum ALT (SGPT) should be performed for the duration of Triglide therapy and therapy should be discontinued if enzyme levels persist above three times the normal limit.
5.4 Serum Creatinine
Elevations in serum creatinine have been reported in patients on fenofibrate. These elevations tend to return to baseline following discontinuation of fenofibrate. The clinical significance of these observations is unknown. Consider monitoring renal function in patients taking Triglide who are at risk for renal impairment, such as the elderly and patients with diabetes. FENOFIBRATE should be avoided in patients with mild or moderate renal impairment. Triglide is contraindicated in patients with severe renal impairment, including those with end-stage renal disease (ESRD) and those receiving dialysis [see Contraindications (4), Use in Specific Populations (8.6),and Clinical Pharmacology (12.3)].
5.5 Cholelithiasis
Fenofibrate, like clofibrate and gemfibrozil, may increase cholesterol excretion into the bile, leading to cholelithiasis. If cholelithiasis is suspected, gallbladder studies are indicated. FENOFIBRATE therapy should be discontinued if gallstones are found.
5.6 Coumarin Anticoagulants
Caution should be exercised when anticoagulants are given in conjunction with FENOFIBRATE because of the potentiation of coumarin-type anti-coagulant effects in prolonging the prothrombin time/International Normalized Ration (PT/INR). The dosage of the anticoagulant should be reduced to maintain the PT/INR at the desired level to prevent bleeding complications. Frequent PT/INR determinations are advisable until it has been definitely determined that the PT/INR has stabilized [see Drug Interactions (7.1)].
5.7 Pancreatitis
Pancreatitis has been reported in patients taking fenofibrate, gemfibrozil, and clofibrate. This occurrence may represent a failure of efficacy in patients with severe hypertriglyceridemia, a direct drug effect, or a secondary phenomenon mediated through biliary tract stone or sludge formation with obstruction of the common bile duct.
5.8 Hematologic Changes
Mild to moderate hemoglobin, hematocrit, and white blood cell decreases have been observed in patients following initiation of fenofibrate therapy. However, these levels stabilize during long-term administration. Thrombocytopenia and agranulocytosis have been reported in individuals treated with fenofibrate. Periodic monitoring of red and white cell counts is recommended during the first 12 months of Triglide administration.
5.9 Hypersensitivity Reactions
Acute Hypersensitivity
Anaphylaxis and angioedema have been reported postmarketing with fenofibrate. In some cases, reactions were life-threatening and required emergency treatment. If a patient develops signs or symptoms of an acute hypersensitivity reaction, advise them to seek immediate medical attention and discontinue fenofibrate.
Delayed Hypersensitivity
Severe cutaneous adverse drug reactions (SCAR), including Stevens-Johnson Syndrome, Toxic Epidermal Necrolysis, and Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS), have been reported postmarketing, occurring days to weeks after initiation of fenofibrate. The cases of DRESS were associated with cutaneous reactions (such as rash or exfoliative dermatitis) and a combination of eosinophilia, fever, systemic organ involvement (renal, hepatic, or respiratory). Discontinue fenofibrate and treat patients appropriately if SCAR is suspected.
5.10 Venothromboembolic Disease
In the FIELD trial, pulmonary embolus (PE) and deep vein thrombosis (DVT) were observed at higher rates in the fenofibrate- than the placebo-treated group. Of 9,795 patients enrolled in FIELD, there were 4,900 in the placebo group and 4,895 in the fenofibrate group. For DVT, there were 48 events (1%) in the placebo group and 67 (1%) in the fenofibrate group (p = 0.074); and for PE, there were 32 (0.7%) events in the placebo group and 53 (1%) in the fenofibrate group (p = 0.022).
In the Coronary Drug Project, a higher proportion of the clofibrate group experienced definite or suspected fatal or nonfatal pulmonary embolism or thrombophlebitis than the placebo group (5.2% vs. 3.3% at five years; p < 0.01).
5.11 Paradoxical Decrease in HDL Cholesterol Levels
There have been postmarketing and clinical trial reports of severe decreases in HDL cholesterol levels (as low as 2 mg/dL) occurring in diabetic and non-diabetic patients initiated on fibrate therapy. The decrease in HDL-C is mirrored by a decrease in apolipoprotein A1. This decrease has been reported to occur within 2 weeks to years after initiation of fibrate therapy. The HDL-C levels remain depressed until fibrate therapy has been withdrawn; the response to withdrawal of fibrate therapy is rapid and sustained. The clinical significance of this decrease in HDL-C is unknown. It is recommended that the HDL-C levels be checked within the first few months after initiation of fibrate therapy. If a severely depressed HDL-C level is detected, fibrate therapy should be withdrawn, and the HDL-C level monitored until it has returned to baseline, and fibrate therapy should not be re-initiated.
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to rates in the clinical studies of another drug and may not reflect rates observed in clinical practice.
Adverse reactions reported by 2% or more of patients treated with fenofibrate (and greater than placebo) during double-blind, placebo-controlled trials are listed in Table 1. Adverse reactions led to discontinuation of treatment in 5.0% of patients treated with fenofibrate and in 3.0% treated with placebo. Increases in liver function tests were the most frequent events, causing discontinuation of fenofibrate treatment in 1.6% of patients in double-blind trials.
Table 1. Adverse Reactions Reported by 2% or More of Patients Treated with Fenofibrate and Greater than Placebo During the Double-Blind, Placebo-Controlled Trials
|
BODY SYSTEM Adverse Reaction | Fenofibrate* (N=439) | Placebo (N=365) |
| BODY AS A WHOLE | ||
| Abdominal Pain | 4.6% | 4.4% |
| Back Pain | 3.4% | 2.5% |
| Headache | 3.2% | 2.7% |
|
DIGESTIVE |
||
| Nausea | 2.3% | 1.9% |
| Constipation | 2.1% | 1.4% |
| METABOLIC AND NUTRITIONAL DISORDERS | ||
| Abnormal Liver Tests | 7.5%** | 1.4% |
| Increased AST | 3.4%** | 0.5% |
| Increased ALT | 3.0% | 1.6% |
| Increased Creatine Phosphokinase | 3.0% | 1.4% |
| RESPIRATORY | ||
| Respiratory Disorder | 6.2% | 5.5% |
| Rhinitis | 2.3% | 1.1% |
*Dosage equivalent to 200 mg fenofibrate capsules, micronized. Dosage comparable to 160 mg Triglide.
**Significantly different from placebo.
Urticaria was seen in 1.1 vs. 0%, and rash in 1.4 vs. 0.8% of fenofibrate and placebo patients respectively in controlled trials.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post approval use of fenofibrate. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure:
myalgia, rhabdomyolysis, pancreatitis, muscle spasms, acute renal failure, hepatitis, cirrhosis, anemia, arthralgia, asthenia, and severely depressed HDL-cholesterol levels. Photosensitivity reactions have occurred days to months after initiation; in some of these cases, patients reported a prior photosensitivity reaction to ketoprofen.
7 DRUG INTERACTIONS
7.1 Coumarin Anticoagulants
Potentiation of coumarin-type anticoagulant effects has been observed with prolongation of the PT/INR.
Caution should be exercised when coumarin anticoagulants are given in conjunction with Triglide. The dosage of the anticoagulants should be reduced to maintain the PT/INR at the desired level to prevent bleeding complications. Frequent PT/INR determinations are advisable until it has been definitely determined that the PT/INR has stabilized [see Warnings and Precautions (5.6)].
7.2 Immunosuppressants
Immunosuppressants such as cyclosporine and tacrolimus can produce nephrotoxicity with decreases in creatinine clearance and rises in serum creatinine, and because renal excretion is the primary elimination route of fibrate drugs including Triglide, there is a risk that an interaction will lead to deterioration of renal function. The benefits and risks of using Triglide with immunosuppressants and other potentially nephrotoxic agents should be carefully considered, and the lowest effective dose employed and renal function monitored.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category C
Safety in pregnant women has not been established. There are no adequate and well controlled studies of fenofibrate in pregnant women. Fenofibrate should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
In female rats given oral dietary doses of 15, 75, and 300 mg/kg/day of fenofibrate from 15 days prior to mating through weaning, maternal toxicity was observed at 0.3 times the maximum recommended human dose (MRHD), based on body surface area comparisons; mg/m2.
In pregnant rats given oral dietary doses of 14, 127, and 361 mg/kg/day from gestation day 6-15 during the period of organogenesis, adverse developmental findings were not observed at 14 mg/kg/day (less than 1 times the MRHD, based on body surface area comparisons; mg/m2). At higher multiples of human doses evidence of maternal toxicity was observed.
In pregnant rabbits given oral gavage doses of 15, 150, and 300 mg/kg/day from gestation day 6-18 during the period of organogenesis and allowed to deliver, aborted litters were observed at 150 mg/kg/day (10 times the MRHD, based on body surface area comparisons: mg/m2). No developmental findings were observed at 15 mg/kg/day (at less than 1 times the MRHD, based on body surface area comparisons; mg/m2).
In pregnant rats given oral dietary doses of 15, 75, and 300 mg/kg/day from gestation day 15 through lactation day 21 (weaning), maternal toxicity was observed at less than 1 times the MRHD, based on body surface area comparisons; mg/m2.
8.3 Nursing Mothers
Fenofibrate should not be used in nursing mothers. A decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
8.5 Geriatric Use
Fenofibric acid is known to be substantially excreted by the kidney, and the risk of adverse reactions to this drug may be greater in patients with impaired renal function. Fenofibric acid exposure is not influenced by age. Since elderly patients have a higher incidence of renal impairment, use of Triglide in the elderly should be made on the basis of renal function [see Contraindications (4), Use in Specific Populations (8.6), and Clinical Pharmacology (12.3)]. Consider monitoring renal function in elderly patients taking FENOFIBRATE.
8.6 Renal Impairment
Patients with severe renal impairment have 2.7-fold higher exposure of fenofibric acid and increased accumulation of fenofibric acid during chronic dosing compared with healthy volunteers. Thus, Triglide is contraindicated in patients with severe renal impairment, including those with end-stage renal disease (ESRD) and those receiving dialysis. In addition, avoid use in patients with mild or moderate renal impairment [see Contraindications (4), Warnings and Precautions (5.4), and Clinical Pharmacology (12.3)].
10 OVERDOSAGE
The use of FENOFIBRATE has not been evaluated in subjects with hepatic impairment [see Contraindications (4) and Clinical Pharmacology (12.3)].
11 DESCRIPTION
FENOFIBRATE Tablets, is a lipid regulating agent available as tablets for oral administration. Each tablet contains 160 mg of fenofibrate.
The chemical name for fenofibrate is 2-[4-(4-chlorobenzoyl) phenoxy] 2-methyl-propanoic acid, 1-methylethyl ester with the following structural formula:
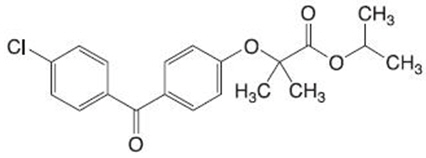
The empirical formula is C20H21O4Cl and the molecular weight is 360.83; fenofibrate is insoluble in water. The melting point is 79° to 82°C. Fenofibrate is a white solid which is stable under ordinary conditions.
Inactive Ingredients: Each tablet contains crospovidone, lactose monohydrate, mannitol, maltodextrin, carboxymethylcellulose sodium, egg lecithin, croscarmellose sodium, sodium lauryl sulfate, colloidal silicon dioxide, magnesium stearate, and monobasic sodium phosphate.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The active moiety of Triglide is fenofibric acid. The pharmacological effects of fenofibric acid in both animals and humans have been extensively studied through oral administration of fenofibrate.
The lipid-modifying effects of fenofibric acid seen in clinical practice have been explained in vivo in transgenic mice and in vitro in human hepatocyte cultures by the activation of peroxisome proliferators activated receptor α (PPARα). Through this mechanism, fenofibrate increases lipolysis and elimination of triglyceride-rich particles from plasma by activating lipoprotein lipase and reducing production of apoprotein C-III (an inhibitor of lipoprotein lipase activity).
The resulting decrease in TG produces an alteration in the size and composition of LDL from small, dense particles (which are thought to be artherogenic due to their susceptibility to oxidation), to large buoyant particles. These larger particles have a greater affinity for cholesterol receptors and are catabolized rapidly. Activation of PPARα also induces an increase in the synthesis of apoproteins A-I, A-II and HDL-cholesterol.
Fenofibrate also reduces serum uric acid levels in hyperuricemic and normal individuals by increasing the urinary excretion of uric acid.
12.2 Pharmacodynamics
A variety of clinical studies have demonstrated that elevated levels of TC, LDL-C, and apo B, an LDL membrane complex, are associated with human atherosclerosis. Similarly, decreased levels of HDL-C and its transport complex, apolipoprotein A (apo AI and apo AII) are associated with the development of atherosclerosis. Epidemiologic investigations have established that cardiovascular morbidity and mortality vary directly with the level of TC, LDL-C, and triglycerides (TG), and inversely with the level of HDL-C. The independent effect of raising HDL-C or lowering TG on the risk of cardiovascular morbidity and mortality has not been determined.
Fenofibric acid, the active metabolite of fenofibrate, produces reductions in total cholesterol, LDL cholesterol, apolipoprotein B, total triglycerides and triglyceride rich lipoprotein (VLDL) in treated patients. In addition, treatment with fenofibrate results in increases in high density lipoprotein (HDL) and apoproteins apo AI and apo AII.
12.3 Pharmacokinetics
FENOFIBRATE 160 mg tablet was shown to have comparable bioavailability to a single dose of 200 mg fenofibrate capsule, micronized. Fenofibrate is a pro-drug of the active chemical moiety fenofibric acid. Fenofibrate is converted by ester hydrolysis in the body to fenofibric acid which is the active constituent measurable in the circulation.
FENOFIBRATE 160 mg tablet was shown to have comparable bioavailability to a single dose of 200 mg fenofibrate capsule, micronized. Fenofibrate is a pro-drug of the active chemical moiety fenofibric acid. Fenofibrate is converted by ester hydrolysis in the body to fenofibric acid which is the active constituent measurable in the circulation.
Distribution: In healthy volunteers, steady-state plasma levels of fenofibric acid were shown to be achieved within a week of dosing and did not demonstrate accumulation across time following multiple dose administration. Serum protein binding was approximately 99% in normal and hyperlipidemic subjects.
Metabolism: Following oral administration, fenofibrate is rapidly hydrolyzed by esterases to the active metabolite, fenofibric acid; no unchanged fenofibrate is detected in plasma. Fenofibric acid is primarily conjugated with glucuronic acid and then excreted in urine. A small amount of fenofibric acid is reduced at the carbonyl moiety to a benzhydrol metabolite which is, in turn, conjugated with glucuronic acid and excreted in urine. In vivo metabolism data indicate that neither fenofibrate nor fenofibric acid undergo oxidative metabolism (e.g., cytochrome P450) to a significant extent.
Elimination: After absorption, fenofibrate is mainly excreted in the urine in the form of metabolites, primarily fenofibric acid and fenofibric acid glucuronide. After administration of radiolabelled fenofibrate, approximately 60% of the dose appeared in the urine and 25% was excreted in the feces. Fenofibric acid is eliminated with a half-life of approximately 16 hours, allowing once daily dosing.
Geriatrics: In elderly volunteers 77 to 87 years of age, the oral clearance of fenofibric acid following a single oral dose of fenofibrate was 1.2 L/h, which compares to 1.1 L/h in young adults. This indicates that a similar dosage regimen can be used in the elderly, without increasing accumulation of the drug or metabolites.
Race: The influence of race on the pharmacokinetics of fenofibrate has not been studied; however, fenofibrate is not metabolized by enzymes known for exhibiting inter-ethnic variability.
Renal Impairment: The pharmacokinetics of fenofibric acid was examined in patients with mild, moderate, and severe renal impairment. Patients with severe renal impairment (creatinine clearance [CrCl ≤ 30 mL/min] < 30 mL/min or estimated glomerular filtration rate [eGFR] <30 mL/min/1.73 m2) showed 2.7-fold increase in exposure for fenofibric acid and increased accumulation of fenofibric acid during chronic dosing compared to that of healthy subjects. Patients with mild to moderate renal impairment (CrCl 30-80 mL/min or eGFR 30-59 mL/min/1.73 m2) had similar exposure but an increase in the half-life for fenofibric acid compared to that of healthy subjects. Based on these findings, avoid use of Triglide in patients with mild or moderate renal impairment. Triglide is contraindicated for patients with severe renal impairment, including end-stage renal disease (ESRD) or those receiving dialysis [see Contraindications (4) and Use in Special Populations (8.6)].
Hepatic Impairment: No pharmacokinetic studies have been conducted in patients having hepatic impairment.
Drug-Drug Interactions:In vitro studies using human liver microsomes indicate that fenofibrate and fenofibric acid are not inhibitors of cytochrome (CYP) P450 isoforms CYP3A4, CYP2D6, CYP2E1, or CYP1A2. They are weak inhibitors of CYP2C8, CYP2C19 and CYP2A6, and mild-to-moderate inhibitors of CYP2C9 at therapeutic concentrations.
Table 2 describes the effects of co-administered drugs on fenofibric acid systemic exposure.
Table 3 describes the effects of fenofibrate on co-administered drugs.
Table 2. Effects of Co-Administered Drugs on Fenofibric Acid Systemic Exposure from Fenofibrate Administration
| Co-Administered Drug | Dosage Regimen of Co-Administered Drug | Dosage Regimen of Fenofibrate | Changes in Fenofibric Acid Exposure | |
| AUC | Cmax | |||
| Lipid-lowering agents | ||||
| Atorvastatin | 20 mg once daily for 10 days | Fenofibrate 160 mg1 once daily for 10 days | ↓2% | ↓4% |
| Pravastatin | 40 mg as a single dose | Fenofibrate 3 × 67 mg2 as a single dose | ↓1% | ↓2% |
| Fluvastatin | 40 mg as a single dose | Fenofibrate 160 mg1 as a single dose | ↓2% | ↓10% |
| Anti-diabetic agents | ||||
| Glimepiride | 1 mg as a single dose | Fenofibrate 145 mg1 once daily for 10 days | ↑1% | ↓1% |
| Metformin | 850 mg three times daily for 10 days | Fenofibrate 54 mg1 three times daily for 10 days | ↓9% | ↓6% |
| Rosiglitazone | 8 mg once daily for 5 days | Fenofibrate 145 mg1 once daily for 14 days | ↑10% | ↑3% |
1TriCor (fenofibrate) oral tablet
2TriCor (fenofibrate) oral micronized capsule
Table 3. Effects of Fenofibrate on Systemic Exposure of Co-Administered Drugs
|
Dosage Regimen of Fenofibrate |
Dosage Regimen of Co-Administered Drug |
Change in Co-Administered Drug Exposure |
||
|---|---|---|---|---|
|
Analyte |
AUC |
Cmax |
||
|
Lipid-lowering agents |
||||
|
Fenofibrate 160 mg1 once daily for 10 days |
Atorvastatin, 20 mg once daily for 10 days |
Atorvastatin |
↓17% |
0% |
|
Fenofibrate 3 × 67 mg2 as a single dose |
Pravastatin, 40 mg as a single dose |
Pravastatin |
↑13% |
↑13% |
|
3α-Hydroxyl-iso-pravastatin |
↑26% |
↑29% |
||
|
Fenofibrate 160 mg1 as a single dose |
Fluvastatin, 40 mg as a single dose |
(+)-3R, 5S-Fluvastatin |
↑15% |
↑16% |
|
Anti-diabetic agents |
||||
|
Fenofibrate 145 mg1 once daily for 10 days |
Glimepiride, 1 mg as a single dose |
Glimepiride |
↑35% |
↑18% |
|
Fenofibrate 54 mg1 three times daily for 10 days |
Metformin, 850 mg three times daily for 10 days |
Metformin |
↑3% |
↑6% |
|
Fenofibrate 145 mg1once daily for 14 days |
Rosiglitazone, 8 mg once daily for 5 days |
Rosiglitazone |
↑6% |
↓1% |
1TriCor (fenofibrate) oral tablet
2TriCor (fenofibrate) oral micronized capsule
13 NON-CLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis: Two dietary carcinogenicity studies have been conducted in rats with fenofibrate. In the first 24-month study, Wistar rats were dosed with fenofibrate at 10, 45 and 200 mg/kg/day, approximately 0.3, 1, and 6 times the maximum recommended human dose (MRHD), based on body surface area comparisons (mg/m2). At a dose of 200 mg/kg/day (at 6 times MRHD), the incidence of liver carcinoma was significantly increased in both sexes. A statistically significant increase in pancreatic carcinomas was observed in males at 1 and 6 times the MRHD; an increase in pancreatic adenomas and benign testicular interstitial cell tumors was observed in males at 6 times the MRHD. In a second 24-month study in a different strain of rats (Sprague-Dawley), doses of 10 and 60 mg/kg/day (0.3 and 2 times the MRHD) produced significant increases in the incidence of pancreatic acinar adenomas in both sexes and increases in testicular interstitial cell tumors in males at 2 times the MRHD.
A 117-week carcinogenicity study was conducted in rats comparing three drugs: fenofibrate 10 and 60 mg/kg/day (0.3 and 2 times the MRHD), clofibrate (400 mg/kg; 2 times the human dose), and gemfibrozil (250 mg/kg; 2 times the human dose, based on mg/m2 surface area). Fenofibrate increased pancreatic acinar adenomas in both sexes. Clofibrate increased hepatocellular carcinomas in males and hepatic neoplastic nodules in females. Gemfibrozil increased hepatic neoplastic nodules in males and females, while all three drugs increased testicular interstitial cell tumors in males.
In a 21-month study in CF-1 mice, fenofibrate 10, 45 and 200 mg/kg/day (approximately 0.2, 1, and 3 times the MRHD on the basis of mg/m2 surface area) significantly increased the liver carcinomas in both sexes at 3 times the MRHD. In a second 18 month study at 10, 60 and 200 mg/kg/day, fenofibrate significantly increased the liver carcinomas in male mice and liver adenomas in female mice at 3 times the MRHD.
Electron microscopy studies have demonstrated peroxisomal proliferation following fenofibrate administration to the rat. An adequate study to test for peroxisome proliferation in humans has not been done, but changes in peroxisome morphology and numbers have been observed in humans after treatment with other members of the fibrate class when liver biopsies were compared before and after treatment in the same individual.
Mutagenesis: Fenofibrate has been demonstrated to be devoid of mutagenic potential in the following tests: Ames, mouse lymphoma, chromosomal aberration and unscheduled DNA synthesis in primary rat hepatocytes.
Impairment of Fertility: In fertility studies rats were given oral dietary doses of fenofibrate, males received 61 days prior to mating and females 15 days prior to mating through weaning which resulted in no adverse effect on fertility at doses up to 300 mg/kg/day (approximately 10 times the MRHD, based on mg/m2 surface area comparisons).
14 CLINICAL STUDIES
14.1 Primary Hypercholesterolemia (Heterozygous Familial and Nonfamilial) and Mixed Dyslipidemia
The effects of fenofibrate at a dose comparable to Triglide 160 mg per day were assessed from four randomized, placebo-controlled, double-blind, parallel-group studies including patients with the following mean baseline lipid values: total-C 306.9 mg/dL; LDL-C 213.8 mg/dL; HDL-C 52.3 mg/dL; triglycerides 191.0 mg/dL. Fenofibrate therapy lowered LDL-C, Total-C, and the LDL-C/HDL-C ratio. Fenofibrate therapy also lowered triglycerides and raised HDL-C (see Table 4).
Table 4. Mean Percent Change in Lipid Parameters at End of Treatment*
| Treatment Group | Total-C | LDL-C | HDL-C | TG |
|---|---|---|---|---|
| Pooled Cohort | ||||
| Mean baseline lipid values (n=646) | 306.9 mg/dL | 213.8 mg/dL | 52.3 mg/dL | 191.0 mg/dL |
| All FEN (n=361) | -18.7%† | -20.6%† | +11.0%† | -28.9%† |
| Placebo (n=285) | -0.4% | -2.2% | +0.7% | +7.7% |
| Baseline LDL-C >160 mg/dL and TG <150 mg/dL | ||||
| Mean baseline lipid values (n=334) | 307.7 mg/dL | 227.7 mg/dL | 58.1 mg/dL | 101.7 mg/dL |
| All FEN (n=193) | -22.4%† | -31.4%† | +9.8%† | -23.5%† |
| Placebo (n=141) | +0.2% | -2.2% | +2.6% | +11.7% |
| Baseline LDL-C >160 mg/dL and TG ≥150 mg/dL | ||||
| Mean baseline lipid values (n=242) | 312.8 mg/dL | 219.8 mg/dL | 46.7 mg/dL | 231.9 mg/dL |
| All FEN (n=126) | -16.8%† | -20.1%† | +14.6%† | -35.9%† |
| Placebo (n=116) | -3.0% | -6.6% | +2.3% | +0.9% |
* Duration of study treatment was 3 to 6 months.
† p=<0.05 vs. placebo
In a subset of the subjects, measurements of apo B were conducted. Fenofibrate treatment significantly reduced apo B from baseline to endpoint as compared with placebo (-25.1% vs. 2.4%, p<0.0001, n=213 and 143 respectively).
14.2 Severe Hypertriglyceridemia
The effects of fenofibrate on serum triglycerides were studied in two randomized, double-blind, placebo-controlled clinical trials of 147 hypertriglyceridemic patients. Patients were treated for eight weeks under protocols that differed only in that one entered patients with baseline triglyceride (TG) levels of 500 to 1500 mg/dL, and the other TG levels of 350 to 500 mg/dL. In patients with hypertriglyceridemia and normal cholesterolemia with or without hyperchylomicronemia, treatment with fenofibrate at dosages equivalent to 160 mg Triglide per day decreased primarily very low density lipoprotein (VLDL) triglycerides and VLDL cholesterol. Treatment of patients with elevated triglycerides often results in an increase of low density lipoprotein (LDL) cholesterol (see Table 5).
Table 5. Effects of Fenofibrate in Patients With Severe Hypertriglyceridemia
| Study 1 |
Placebo |
Fenofibrate** |
||||||
| Baseline TG levels 350 to 499 mg/dL |
N |
Baseline (Mean) |
Endpoint (Mean) |
% Change (Mean) |
N |
Baseline (Mean) |
Endpoint (Mean) |
% Change (Mean) |
| Triglycerides |
28 |
449 |
450 |
-0.5 |
27 |
432 |
223 |
-46.2* |
| VLDL Triglycerides |
19 |
367 |
350 |
2.7 |
19 |
350 |
178 |
-44.1* |
| Total Cholesterol |
28 |
255 |
261 |
2.8 |
27 |
252 |
227 |
-9.1* |
| HDL Cholesterol |
28 |
35 |
36 |
4 |
27 |
34 |
40 |
19.6* |
| LDL Cholesterol |
28 |
120 |
129 |
12 |
27 |
128 |
137 |
14.5 |
| VLDL Cholesterol |
27 |
99 |
99 |
5.8 |
27 |
92 |
46 |
-44.7* |
| Study 2 |
Placebo |
Fenofibrate** |
||||||
| Baseline TG levels 500 to 1500 mg/dL |
N |
Baseline (Mean) |
Endpoint (Mean) |
% Change (Mean) |
N |
Baseline (Mean) |
Endpoint (Mean) |
% Change (Mean) |
| Triglycerides |
44 |
710 |
750 |
7.2 |
48 |
726 |
308 |
-54.5* |
| VLDL Triglycerides |
29 |
537 |
571 |
18.7 |
33 |
543 |
205 |
-50.6* |
| Total Cholesterol |
44 |
272 |
271 |
0.4 |
48 |
261 |
223 |
-13.8* |
| HDL Cholesterol |
44 |
27 |
28 |
5.0 |
48 |
30 |
36 |
22.9* |
| LDL Cholesterol |
42 |
100 |
90 |
-4.2 |
45 |
103 |
131 |
45.0* |
| VLDL Cholesterol |
42 |
137 |
142 |
11.0 |
45 |
126 |
54 |
-49.4* |
| *=p<0.05 vs. placebo **Dosage comparable to 160 mg Triglide |
||||||||
The effect of Triglide on cardiovascular morbidity and mortality has not been determined.
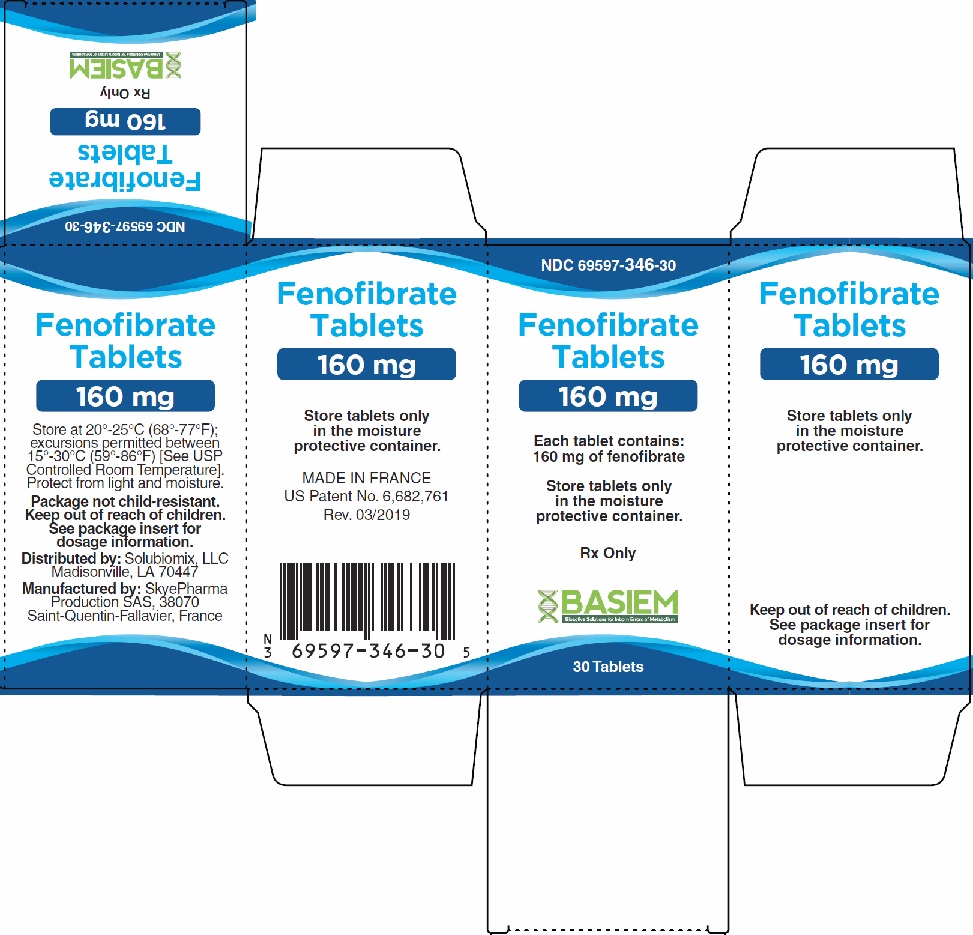
16 HOW SUPPLIED/STORAGE AND HANDLING
The FENOFIBRDATE tablets are supplied as follows:
NDC: 69597-346-30: bottles of 30 tablets. 160 mg, off-white round tablets, debossed "FH 160".
Only dispense FENOFIBRATE tablets in the original manufacturer bottle with the original desiccant cap. Do not repackage Triglide tablets into standard amber pharmacy vials.
Store at 20°-25°C (68°-77°F); excursions permitted to 15°-30°C (59°-86°F) [See USP Controlled Room Temperature]. Protect from light and moisture. Store tablets only in the moisture protective container.
17 PATIENT COUNSELING INFORMATION
Instruct patients to take FENOFIBRATE once daily at the prescribed dose, swallowing each tablet whole. Additionally, instruct patients not to take chipped or broken tablets.
Instruct patients to keep FENOFIBRATE in the original bottle to protect from moisture. The bottle contains a desiccant in the cap to protect tablets from moisture. Tablets should not be stored or placed in any other container, such as pill boxes or pill organizers.
Instruct patients to continue following an appropriate lipid-modifying diet while taking FENOFIBRATE.
Advise patients not to use FENOFIBRATE if they have a known hypersensitivity to fenofibrate or fenofibric acid.
Advise patients to promptly report any unexplained muscle pain, tenderness, or weakness, particularly if accompanied by malaise or fever; onset of abdominal pain or other symptoms consistent with gallstones or pancreatitis; or any other new symptoms.
Inform patients that if they are taking coumarin anticoagulants, FENOFIBRATE may increase their anti-coagulant effect and that increased monitoring may be necessary.
Advise patients regarding medications that should not be taken in combination with FENOFIBRATE or that may necessitate increased monitoring. Instruct patients to inform their health care providers regarding all medications, supplements, and herbal preparations that they are taking.
Manufactured for:
Basiem, LLC
Madisonville, LA 70447
Manufactured by:
SkyePharma Production SAS
38070 Saint-Quentin-Fallavier, France
Revised: 03/2019
| FENOFIBRATE
fenofibrate tablet |
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| Labeler - Basiem (079686680) |