VETROPOLYCIN- bacitracin zinc, neomycin sulfate, and polymyxin b sulfate ointment
Vetropolycin by
Drug Labeling and Warnings
Vetropolycin by is a Animal medication manufactured, distributed, or labeled by Dechra Veterinary Products, Altaire Pharmaceuticals, Inc., Xellia Pharmaceuticals Inc., Pharmacia and Upjohn Company, Xellia Pharmaceuticals AS. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- SPL UNCLASSIFIED SECTION
- DESCRIPTION
-
VETERINARY INDICATIONS
INDICATIONS: For the treatment of superficial bacterial infections of the eyelid and conjunctiva in dogs and cats when due to organisms susceptible to the antibiotics contained in the ointment.
Laboratory tests should be conducted including in vitro culturing and susceptibility tests on samples collected prior to treatment.
- DOSAGE & ADMINISTRATION
-
WARNINGS
WARNING: Do not use this product as a pre-surgical ocular lubricant. Adverse reactions of ocular irritation and corneal ulceration have been reported in association with such use.
Serious hypersensitivity (anaphylactic) reactions have been reported in cats within 4 hours of application of antibiotic ophthalmic preparations. Some of these reactions have resulted in death.
- SAFE HANDLING WARNING
-
PRECAUTIONS
PRECAUTIONS: Sensitivity to this ophthalmic ointment is rare, however, if a reaction occurs, discontinue use of the preparation. The prolonged use of antibiotic-containing preparations may result in overgrowth of nonsusceptible organisms including fungi.
Appropriate measures should be taken if this occurs.
If infection does not respond to treatment in two or three days, the diagnosis and therapy should be re-evaluated.
Care should be taken to not contaminate the applicator tip of the tube during the application of the preparation. Do not allow the applicator tip to come in contact with any tissue.
-
ADVERSE REACTIONS
ADVERSE REACTIONS: Itching, burning or inflammation may occur in animals sensitive to the product. Discontinue use in such cases.
For a copy of the Safety Data Sheet (SDS), or to report adverse reactions, call Dechra Veterinary Products at (866) 933-2472.
For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS, or http://www.fda.gov/AnimalVeterinary/SafetyHealth
-
MECHANISM OF ACTION
ACTIONS: The three antibiotics present in Vetropolycin (neomycin and polymyxin B sulfates and bacitracin zinc ophthalmic ointment) Veterinary Ophthalmic Ointment provide a broad spectrum of activity against the gram-positive and gram-negative bacteria commonly involved in superficial infections of the eyelid and conjunctiva.
Bacitracin is effective against gram-positive bacteria including hemolytic and non-hemolytic streptococci and staphylococci.
Resistant strains rarely develop.
Neomycin is effective against both gram-positive and gram-negative bacteria including staphylococci, Escherichia coli and Haemophilus influenzae and many strains of Proteus and Pseudomonas.
Polymyxin B is bactericidal to gram-negative bacteria especially Pseudomonas. No resistant strains have been found to develop in vivo.
- STORAGE AND HANDLING
- HOW SUPPLIED
- SPL UNCLASSIFIED SECTION
-
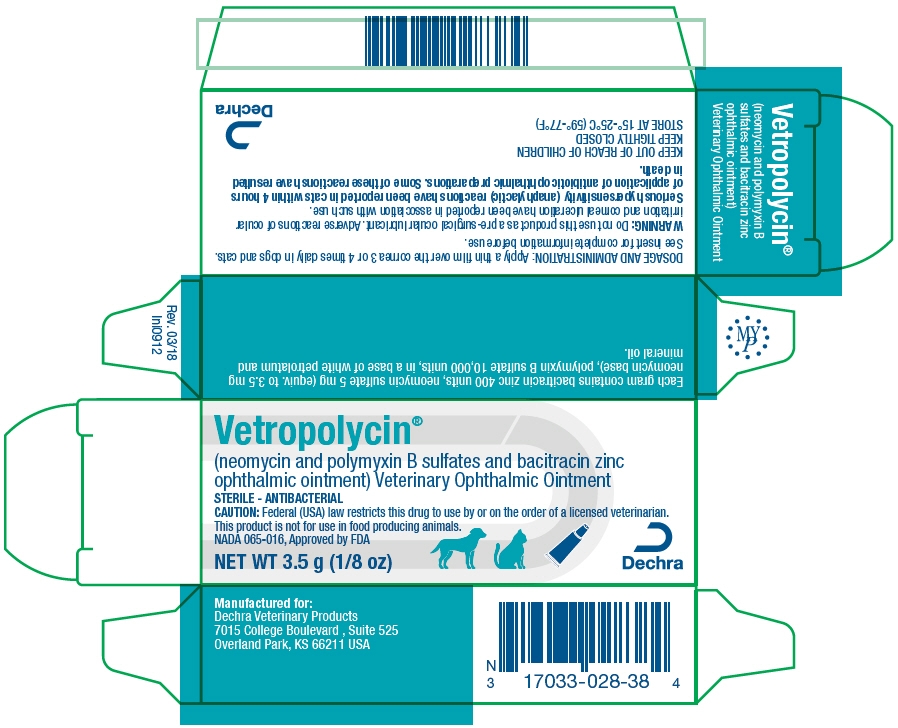
PRINCIPAL DISPLAY PANEL - 3.5 g Tube Carton
Vetropolycin®
(neomycin and polymyxin B sulfates and bacitracin zinc
ophthalmic ointment) Veterinary Ophthalmic OintmentSTERILE - ANTIBACTERIAL
CAUTION: Federal (USA) law restricts this drug to use by or on the order of a licensed veterinarian.
This product is not for use in food producing animals.
NADA 065-016, Approved by FDANET WT 3.5 g (1/8 oz)
Dechra
-
INGREDIENTS AND APPEARANCE
VETROPOLYCIN
bacitracin zinc, neomycin sulfate, and polymyxin b sulfate ointmentProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 17033-028 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Bacitracin Zinc (UNII: 89Y4M234ES) (BACITRACIN - UNII:58H6RWO52I) BACITRACIN 400 [USP'U] in 1 g Neomycin Sulfate (UNII: 057Y626693) (NEOMYCIN - UNII:I16QD7X297) NEOMYCIN 5 mg in 1 g Polymyxin B Sulfate (UNII: 19371312D4) (POLYMYXIN B - UNII:J2VZ07J96K) POLYMYXIN B 10000 [USP'U] in 1 g Inactive Ingredients Ingredient Name Strength mineral oil (UNII: T5L8T28FGP) petrolatum (UNII: 4T6H12BN9U) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 17033-028-38 1 in 1 CARTON 1 3.5 g in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA065016 04/10/2014 Labeler - Dechra Veterinary Products (362142734) Establishment Name Address ID/FEI Business Operations Altaire Pharmaceuticals, Inc. 786790378 MANUFACTURE Establishment Name Address ID/FEI Business Operations Xellia Pharmaceuticals Inc. 529171201 API MANUFACTURE Establishment Name Address ID/FEI Business Operations Pharmacia and Upjohn Company 618054084 API MANUFACTURE Establishment Name Address ID/FEI Business Operations Xellia Pharmaceuticals AS 305814345 API MANUFACTURE
Trademark Results [Vetropolycin]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 VETROPOLYCIN 72373558 0932496 Dead/Cancelled |
JOHNSON & JOHNSON 1970-10-16 |
 VETROPOLYCIN 72206428 0804537 Live/Registered |
DOW CHEMICAL COMPANY, THE 1964-11-18 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.