ALCAFTADINE solution/ drops
alcaftadine by
Drug Labeling and Warnings
alcaftadine by is a Otc medication manufactured, distributed, or labeled by Alembic Pharmaceuticals Inc., Gland Pharma Limited. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- ACTIVE INGREDIENT
- PURPOSE
- USE
- WARNINGS
- DO NOT USE
- WHEN USING THIS PRODUCT
- STOP USE AND ASK DOCTOR IF
- KEEP OUT OF REACH OF CHILDREN
- DIRECTIONS
- OTHER INFORMATION
- INACTIVE INGREDIENTS
- QUESTIONS OR COMMENTS?
-
PRINCIPAL DISPLAY PANEL
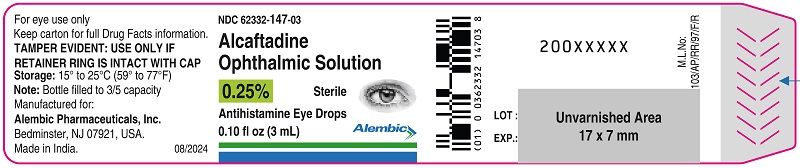
Container Label - 0.25% (3 mL)
NDC: 62332-147-03
Alcaftadine Ophthalmic Solution, 0.25%
Antihistamine Eye Drops
Eye Allergy Itch Relief
Sterile
0.10 fl oz (3 mL)
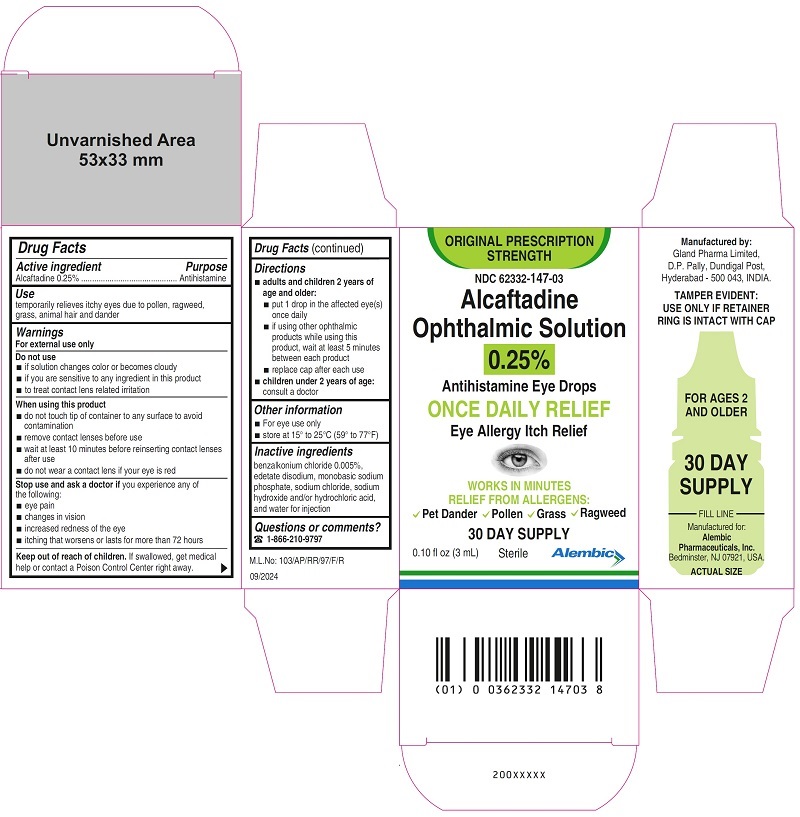
Carton Label - 0.25% (3 mL)
NDC: 62332-147-03
ORIGINAL PRESCRIPTION STRENGTH
Alcaftadine Ophthalmic Solution, 0.25%
Antihistamine Eye Drops
ONCE DAILY RELIEF
Eye Allergy Itch Relief
Sterile
30 DAY SUPPLY
0.10 fl oz (3 mL)
-
INGREDIENTS AND APPEARANCE
ALCAFTADINE
alcaftadine solution/ dropsProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 62332-147 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCAFTADINE (UNII: 7Z8O94ECSX) (ALCAFTADINE - UNII:7Z8O94ECSX) ALCAFTADINE 2.5 mg in 1 mL Inactive Ingredients Ingredient Name Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) EDETATE DISODIUM (UNII: 7FLD91C86K) SODIUM PHOSPHATE, MONOBASIC (UNII: 3980JIH2SW) WATER (UNII: 059QF0KO0R) SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM HYDROXIDE (UNII: 55X04QC32I) HYDROCHLORIC ACID (UNII: QTT17582CB) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 62332-147-03 1 in 1 CARTON 10/02/2024 1 3 mL in 1 BOTTLE; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA209290 10/02/2024 Labeler - Alembic Pharmaceuticals Inc. (079288842) Establishment Name Address ID/FEI Business Operations Gland Pharma Limited 918601238 MANUFACTURE(62332-147)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.