These highlights do not include all the information needed to use MAGNESIUM SULFATE IN 5% DEXTROSE INJECTION safely and effectively. See full prescribing information for MAGNESIUM SULFATE IN 5% DEXTROSE INJECTION. MAGNESIUM SULFATE IN DEXTROSE injection, for intravenous useInitial U.S. Approval: 1941
Magnesium Sulfate in Dextrose by
Drug Labeling and Warnings
Magnesium Sulfate in Dextrose by is a Prescription medication manufactured, distributed, or labeled by Mylan Institutional LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
MAGNESIUM SULFATE IN DEXTROSE- magnesium sulfate heptahydrate injection, solution
Mylan Institutional LLC
----------
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use MAGNESIUM SULFATE IN 5% DEXTROSE INJECTION safely and effectively. See full prescribing information for MAGNESIUM SULFATE IN 5% DEXTROSE INJECTION.
MAGNESIUM SULFATE IN DEXTROSE injection, for intravenous use Initial U.S. Approval: 1941 INDICATIONS AND USAGEDOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHSSupplied in premixed single-dose bags: (3)
WARNINGS AND PRECAUTIONS
ADVERSE REACTIONSThe most common adverse reactions are flushing, sweating, hypotension, depressed reflexes, flaccid paralysis, hypothermia, circulatory collapse, cardiac and central nervous system (CNS) depression proceeding to respiratory paralysis and hypocalcemia. Bradycardia, pulmonary edema, decreased respiratory rate, lethargy, sedation, somnolence, visual disturbances, and hypermagnesemia are also reported (6) To report SUSPECTED ADVERSE REACTIONS, contact Mylan at 1-877-446-3679 (1-877-4-INFO-RX) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. DRUG INTERACTIONS
USE IN SPECIFIC POPULATIONSPatients with severe renal impairment and/or a urine output less than 100 mL every 4 hours are at greater risk for increased magnesium concentrations that may lead to toxicity (8.6) See 17 for PATIENT COUNSELING INFORMATION. Revised: 11/2022 |
FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
Magnesium Sulfate in 5% Dextrose Injection is indicated for:
- Prevention of eclampsia in patients with preeclampsia
- Treatment of seizures and prevention of recurrent seizures in patients with eclampsia
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Instructions
Magnesium Sulfate in 5% Dextrose Injection is:
- A clear solution. Visually inspect Magnesium Sulfate in 5% Dextrose Injection for particulate matter and discoloration prior to administration. Do not administer unless solution is clear and colorless.
- For intravenous use only
- Administered via intravenous infusion pump
Magnesium Sulfate in 5% Dextrose Injection does not require dilution prior to intravenous administration.
After removing the overwrap, check for minute leaks by squeezing the container fully. Do not administer Magnesium Sulfate in 5% Dextrose Injection if there is a leak or there is greater than 2 mL of water in the overwrap [see Description (11)].
Do not administer Magnesium Sulfate in 5% Dextrose Injection with incompatible drugs through the same intravenous line [see Dosage and Administration (2.4)]. Do not use Magnesium Sulfate in 5% Dextrose Injection in series connections.
2.2 Recommended Dosage
- The recommended loading dosage of Magnesium Sulfate in 5% Dextrose Injection in patients with eclampsia or preeclampsia is 4 grams to 6 grams over 15 minutes followed by a recommended maintenance dosage of 1 grams to 2 grams every hour.
- Obtain serum magnesium concentrations and assess clinical status to adjust the dosage.
- In patients with eclampsia, consider targeting the maintenance dosage to achieve serum magnesium concentrations of 3 mg to 6 mg per 100 mL (2.5 to 5 mEq per liter). For patients with recurrent eclampsia, consider giving an additional 2 gram intravenous bolus.
- For patients with eclampsia, therapy should continue until seizures cease.
- The maximum recommended dosage is 30 grams to 40 grams of magnesium sulfate over 24 hours.
Administration of Magnesium Sulfate in 5% Dextrose Injection beyond 5 to 7 days is not recommended [see Warnings and Precautions (5.1)].
2.3 Dosage in Patients with Severe Renal Impairment and/or Oliguria
- In patients with severe renal impairment and/or a urine output less than 0.5 mL/kg/hour, initiate Magnesium Sulfate in 5% Dextrose Injection with a 4 gram loading dose followed by a maintenance dosage of 1 gram every hour.
- Titrate the magnesium sulfate maintenance dosage to maintain concentrations in the target range through frequent monitoring of magnesium concentrations and observation for clinical signs of magnesium toxicity (e.g., facial edema, diminished strength of deep tendon reflexes, respiratory depression). A lower maintenance dosage requirement is likely in these patients.
Do not exceed the maximum recommended dosage of 20 grams of Magnesium Sulfate in 5% Dextrose Injection over 48 hours.
2.4 Drug Incompatibilities
Magnesium Sulfate in 5% Dextrose Injection is not compatible with administration of a variety of solutions and forms precipitates of magnesium salts. Before using Magnesium Sulfate in 5% Dextrose Injection with another parenteral product, investigate potential incompatibilities. Incompatible products that should not be coadministered include salicylates and alkali carbonates.
3 DOSAGE FORMS AND STRENGTHS
Magnesium Sulfate in 5% Dextrose Injection, USP is a clear and colorless solution supplied in single-dose bags:
-
grams per mL (1%):
- 100 mL bag containing 1 gram of Magnesium Sulfate in 5% Dextrose Injection
Each 100 mL contains 5 grams of dextrose monohydrate in Water for Injection.
4 CONTRAINDICATIONS
Magnesium Sulfate in 5% Dextrose Injection is contraindicated in patients:
- with heart block or myocardial damage
- in diabetic coma
- with myasthenia gravis [see Warnings and Precautions (5.6)]
5 WARNINGS AND PRECAUTIONS
5.1 Fetal-Neonatal Toxicity with Prolonged Use
Continuous administration of magnesium sulfate beyond 5 to 7 days in pregnant women can lead to hypocalcemia and bone abnormalities in the developing fetus, including skeletal demineralization and osteopenia. In addition, cases of neonatal fracture have been reported.
Neonates of women receiving Magnesium Sulfate in 5% Dextrose Injection (especially with prolonged maternal use) are at risk for magnesium toxicity including hyporeflexia, hypotonia, and respiratory depression. There is one reported case of neonatal death as the result of magnesium toxicity after transplacental exposure.
The shortest duration of magnesium sulfate treatment that can lead to fetal harm is not known. Administration of Magnesium Sulfate in 5% Dextrose Injection beyond 5 to 7 days is not recommended.
5.2 Risk of Magnesium Toxicity
Patients receiving Magnesium Sulfate in 5% Dextrose Injection are at risk for magnesium toxicity including respiratory depression, acute renal failure and rarely, pulmonary edema.
Monitor clinical signs of magnesium toxicity (for example, facial edema, diminished strength of deep tendon reflexes, respiratory depression) and magnesium concentrations during infusions of Magnesium Sulfate in 5% Dextrose Injection. Clinical indications of a safe dosage regimen include the presence of the patellar reflex (knee jerk) and absence of respiratory depression (approximately 16 breaths or more per minute). Serum magnesium concentrations usually sufficient to control convulsions range from 3 to 6 mg per 100 mL (2.5 to 5 mEq per liter). The strength of the deep tendon reflexes begins to diminish when serum magnesium concentrations exceed 4 mEq per liter. Reflexes may be absent at concentration of 10 mEq per liter, at which point respiratory paralysis is a potential hazard. An injectable calcium salt should be immediately available to counteract the potential hazards of magnesium toxicity in patients with preeclampsia and eclampsia. If there is significant magnesium toxicity, stop the Magnesium Sulfate in 5% Dextrose Injection infusion and recheck serum magnesium concentration.
Patients with renal impairment are at greater risk of magnesium toxicity because magnesium is excreted by the body solely by the kidneys [see Use in Specific Populations (8.6)]. Urine output should be maintained at a level of 100 mL per 4 hours. Monitoring serum magnesium levels and the patient’s clinical status is essential to avoid the consequences of overdosage in patients with preeclampsia. Discontinuation of the magnesium infusion is recommended when urine output is less than 100 mL every 4 hours to avoid magnesium toxicity, especially if serum creatinine is increasing progressively.
5.3 Risk of Elevated Blood Glucose
Solutions containing dextrose should be used with caution in patients with known prediabetes or diabetes mellitus given the risk of elevated blood glucose.
5.4 Co-administration with Unapproved Tocolytics
Do not use Magnesium Sulfate in 5% Dextrose Injection with unapproved tocolytics (e.g., beta adrenergic agents such as terbutaline, or with calcium channel blockers such as nifedipine). Serious adverse events including pulmonary edema and hypotension have occurred [see Drug Interactions (7)].
5.5 Aluminum Toxicity
Magnesium Sulfate in 5% Dextrose Injection contains aluminum that may be toxic (Magnesium Sulfate in 5% Dextrose Injection contains less than 25 mcg/L of aluminum). Aluminum may reach toxic concentrations with prolonged parenteral administration in patients with renal impairment.
Patients with renal impairment who receive parenteral concentrations of aluminum at greater than 4 to 5 mcg/kg/day, accumulate aluminum at concentrations associated with central nervous system and bone toxicity. Tissue loading may occur at even lower rates of administration.
5.6 Exacerbation of Myasthenia Gravis
Magnesium Sulfate in 5% Dextrose Injection is contraindicated in patients with known myasthenia gravis.
Use of magnesium sulfate in patients with underlying myasthenia gravis can precipitate a myasthenic crisis. Myasthenic crisis is a life-threatening condition characterized by neuromuscular respiratory failure. Symptoms of myasthenic crisis may include difficulty swallowing, ptosis, facial droop, weakness and/or difficulty breathing that may require intubation.
If myasthenic crisis is suspected, discontinue use of Magnesium Sulfate in 5% Dextrose Injection immediately. Secure the patient’s airway. Consider intensive care unit admission and elective intubation, if respiratory failure is anticipated. Once the airway is secure, confirm the diagnosis. Therapies include plasmapheresis and plasma exchange or intravenous immunoglobulin (IVIG) and immunomodulating therapy in addition to high-dose glucocorticoids.
6 ADVERSE REACTIONS
The following adverse reactions have been identified in clinical studies or postmarketing reports. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
|
Cardiovascular: |
hypotension, circulatory collapse, cardiac depression including bradycardia |
|
Central Nervous System: |
central nervous system depression leading to respiratory paralysis, visual disturbances, flushing, sweating, hypothermia |
|
Metabolic: |
hypocalcemia with signs of tetany, hypermagnesemia |
|
Neurologic: |
lethargy, sedation, somnolence, myasthenic crisis |
|
Neuromuscular: |
depressed deep tendon reflexes, flaccid paralysis |
|
Pulmonary: |
decreased respiratory rate, pulmonary edema |
7 DRUG INTERACTIONS
Table 1 presents the potential clinical impact of medications that may be commonly administered concomitantly with Magnesium Sulfate in 5% Dextrose Injection in the clinical setting.
|
|
|
|
Neuromuscular Blocking Agents |
|
|
Clinical Impact: |
|
|
Intervention: |
|
|
Examples: |
|
|
Narcotics and/or Propofol |
|
|
Clinical Impact: |
|
|
Intervention: |
|
|
Examples: |
|
|
Dihydropyridine Calcium Channel Blockers |
|
|
Clinical Impact: |
|
|
Intervention: |
|
|
Examples: |
|
|
Drugs that May Induce Magnesium Loss |
|
|
Clinical Impact: |
|
|
Intervention: |
|
|
Examples: |
|
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Magnesium Sulfate in 5% Dextrose Injection is indicated in pregnant women for the prevention of eclampsia in women with preeclampsia and the treatment of seizures and prevention of recurrent seizures in women with eclampsia. Fetal, neonatal, and maternal risks are discussed throughout the labeling.
Clinical Considerations
Labor or Delivery:
Magnesium Sulfate in 5% Dextrose Injection is not approved for the treatment of pre-term labor.
Administration of Magnesium Sulfate in 5% Dextrose Injection to pregnant women longer than 5 to 7 days may lead to hypocalcemia and bone abnormalities in the developing fetus, including skeletal demineralization and osteopenia [see Warnings and Precautions (5.1)].
8.2 Lactation
The use of intravenous magnesium in pregnant women increases human milk magnesium concentrations only slightly and oral absorption of magnesium by the infant is poor. The effect of intravenous magnesium on milk production is unknown. The developmental and health benefits to the neonate of breastfeeding should be considered along with the mother’s clinical need for Magnesium Sulfate in 5% Dextrose Injection and any potential adverse effects on the breastfed infant from Magnesium Sulfate in 5% Dextrose Injection or from the underlying maternal condition.
8.4 Pediatric Use
The safety and effectiveness of Magnesium Sulfate in 5% Dextrose Injection have been established for the prevention of eclampsia in adolescents with preeclampsia and the treatment of seizures and prevention of recurrent seizures in adolescents with eclampsia. Dosing recommendation in pregnant adolescent patients are the same as for pregnant adult patients [see Dosage and Administration (2.2)].
8.6 Renal Impairment
Magnesium is excreted solely by the kidneys. Patients with severe renal impairment (urine output less than 100 mL per 4 hours) are at greater risk for increased magnesium concentrations that may lead to magnesium toxicity [see Warnings and Precautions (5.2) and Clinical Pharmacology (12.3)]. In patients with severe renal impairment, dosage reduction is recommended and the maximum recommended dosage is lower than patients with normal renal function [see Dosage and Administration (2.3)].
10 OVERDOSAGE
Manifestations of magnesium toxicity include a drop in blood pressure, difficulty breathing, and disappearance of the patellar reflex. As serum magnesium rises above 4 mEq per liter, the deep tendon reflexes decrease. As the serum magnesium level approaches 10 mEq per liter, the tendon reflexes disappear and respiratory paralysis may occur [see Warnings and Precautions (5.2)]. Other signs and symptoms of magnesium overdosage include flushing, sweating, hypotension, weakness, hypothermia, circulatory collapse, cardiac and central nervous system depression proceeding to respiratory paralysis, cardiac arrest, and prolongation of PR and QRS intervals. Patients with renal impairment and underlying neuromuscular diseases such as myasthenia gravis may experience magnesium intoxication at lower magnesium concentrations (Magnesium Sulfate in 5% Dextrose Injection is contraindicated in patients with myasthenia gravis).
If patient is experiencing magnesium toxicity, immediately discontinue Magnesium Sulfate in 5% Dextrose Injection. Artificial respiration may be required. Administer an injectable calcium salt to counteract the potential hazards of magnesium toxicity [see Warnings and Precautions (5.2)].
Hypermagnesemia in the newborn (after administration of Magnesium Sulfate in 5% Dextrose Injection to the mother) may require resuscitation and assisted ventilation via endotracheal intubation or intermittent positive pressure ventilation as well as intravenous calcium.
11 DESCRIPTION
Magnesium Sulfate in 5% Dextrose Injection, USP is a sterile, nonpyrogenic, clear, colorless solution of magnesium sulfate heptahydrate and dextrose in water for injection for intravenous use. Each 100 mL contains 1 gram of magnesium sulfate heptahydrate and dextrose monohydrate 5 grams in water for injection [see How Supplied/Storage and Handling (16)]. Magnesium Sulfate in 5% Dextrose Injection, USP may contain sulfuric acid and/or sodium hydroxide for pH adjustment. The pH is 4.5 (3.5 to 6.5).
Magnesium Sulfate, USP heptahydrate is chemically known as sulfuric acid magnesium salt (1:1), heptahydrate and chemically designated MgSO4 7H2O, with a molecular weight of 246.47. It occurs as small colorless crystals freely soluble in water.
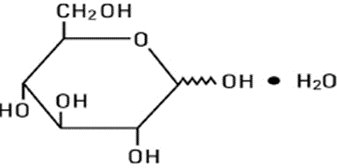
Dextrose, USP is chemically designated D-glucose, monohydrate, a hexose sugar freely soluble in water. The molecular formula is C6H12O6 H2O and the molecular weight is 198.17. It has the following structural formula:
Water for Injection, USP is chemically designated H2O.
Water can permeate from inside the flexible plastic container (polyvinylchloride) into the overwrap [see Dosage and Administration (2.1)] but not in amounts sufficient to affect the solution significantly. Solutions in contact with the plastic container may leach out certain chemical components from the plastic in very small amounts; however, biological testing was supportive of the safety of the plastic container materials. Exposure to temperatures above 25°C (77°F) during transport and storage will lead to minor losses in moisture content. Higher temperatures lead to greater losses. It is unlikely that these minor losses will lead to clinically significant changes within the expiration period.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Magnesium prevents seizures in patients with preeclampsia and controls seizures in patients with eclampsia by blocking neuromuscular transmission and decreasing the amount of acetylcholine liberated at the end plate by the motor nerve impulse. Magnesium has a depressant effect on the central nervous system [see Drug Interactions (7)]. Magnesium acts peripherally to produce vasodilation.
12.2 Pharmacodynamics
With intravenous administration of magnesium sulfate the onset of anticonvulsant action is immediate and lasts about 30 minutes. The estimated magnesium concentration (above baseline) required to elicit half-maximum effect (EC50) on systolic and diastolic blood pressure in pregnant women with preeclampsia that received intravenous magnesium sulfate therapy was reported to be 1.5 and 1.8 mEq per liter (1.9 and 2.2 mg per dL), respectively, in a published study. Effective anticonvulsant serum concentrations range from 2.5 to 7.5 mEq per liter.
Drug Interaction Studies
The following information is based upon published case reports and clinical studies that could not be confirmed by an adequately controlled study, but still warrant consideration given the potential risks involved [see Drug Interactions (7)].
Neuromuscular Blocking Agents
Potentiation and prolongation of neuromuscular blockade requiring modification of the neuromuscular blocking agent dosage and/or increased reversal agent requirements were reported in preeclamptic women who received magnesium sulfate treatment who underwent subsequent surgery (for example, caesarian section) with anesthesia that included either a depolarizing (d-tubocurarine, succinylcholine) or nondepolarizing neuromuscular blocking agent (vecuronium, rocuronium).
Narcotics and/or Propofol
Potentiation and prolongation of analgesic and/or sedative effects as well as a reduced requirement for an intravenous narcotic (fentanyl, sufentanil, tramadol), intrathecal narcotic (fentanyl), and/or intravenous propofol was reported in magnesium sulfate treated patients who required surgery or intensive care that also included narcotic and/or propofol therapy.
Dihydropyridine Calcium Channel Blockers
An exaggerated hypotensive response (blood pressure 80 to 93/49 to 60 mm Hg) was reported in preeclamptic women who received oral nifedipine in addition to magnesium sulfate treatment. Blood pressure returned to previous levels within approximately 30 minutes with supportive care.
12.3 Pharmacokinetics
Distribution
Approximately 1 to 2% of total body magnesium is located in the extracellular fluid space. Magnesium is 30% bound to albumin.
Elimination
The average half-life and systemic clearance of magnesium sulfate in preeclamptic women is approximately 4 to 5 hours and 4 to 5 liters per hour, respectively.
Specific Populations
Patients with Renal Impairment
Plasma magnesium concentrations of 7 to 12.3 mEq per liter (8.6 to 15.1 mg per dL) were reported in preeclamptic women with a urine output less than 100 mL per 4 hours that received 20 grams of magnesium sulfate intravenously over 2 to 8 hours in a published study [see Dosage and Administration (2.3) and Use in Specific Populations (8.6)].
16 HOW SUPPLIED/STORAGE AND HANDLING
Magnesium Sulfate in 5% Dextrose Injection, USP is a clear, colorless solution supplied in single-dose flexible plastic containers (see Table 2).
|
|
||||||
|
Magnesium Sulfate* Concentration |
NDC Number |
Container Size |
Total Magnesium Sulfate* |
Total Magnesium Ion |
Magnesium Ion Concentration |
Osmolarity† |
|
0.01 grams/mL (1%) |
67457-567-00 |
100 mL |
1 gram |
8.1 mEq |
8.1 mEq/100 mL |
333 mOsmol/liter |
Storage
Store at 20° to 25°C (68° to 77°F). [See USP Controlled Room Temperature.] Protect from freezing.
17 PATIENT COUNSELING INFORMATION
Magnesium Sulfate in 5% Dextrose Injection is typically administered to pregnancy women in emergent situations. When feasible, advise the patient and family of the following:
Fetal-Neonatal Toxicity Reported With Prolonged Use
Continuous administration of Magnesium Sulfate in 5% Dextrose Injection in pregnant women beyond 5 to 7 days can lead to hypocalcemia and bone abnormalities in the developing fetus, including skeletal demineralization and osteopenia. In addition, cases of neonatal fracture have been reported [see Warnings and Precautions (5.1)].
Risk of Magnesium Toxicity
Pregnant women receiving Magnesium Sulfate in 5% Dextrose Injection are at risk for magnesium toxicity, including facial edema, diminished strength of deep tendon reflexes, and respiratory depression [see Warnings and Precautions (5.2)].
Manufactured for:
Mylan Institutional LLC
Morgantown, WV 26505 U.S.A.
Manufactured by:
Terumo BCT Ltd
Larne, United Kingdom
NOVEMBER 2022
PRINCIPAL DISPLAY PANEL - 100 mL
NDC: 67457-567-00
100 mL
Magnesium Sulfate
in 5% Dextrose
Injection, USP
(0.081 mEq Mg++/mL)
1 g/100 mL
10 mg/mL
1 g TOTAL
For Intravenous Use
Each 100 mL contains: Magnesium sulfate heptahydrate 1 g (equivalent to 8.1 mEq magnesium) and dextrose monohydrate 5 g in Water for Injection. May contain sulfuric acid and/or sodium hydroxide for pH adjustment.
pH 4.5 (3.5 to 6.5) 333 mOsmol/Liter (CALC.)
Single-Dose Container. DISCARD UNUSED PORTION.
RECOMMENDED DOSAGE: SEE PRESCRIBING INFORMATION. STERILE, NONPYROGENIC. USE ONLY IF SOLUTION IS CLEAR AND CONTAINER IS UNDAMAGED. MUST NOT BE USED IN SERIES CONNECTIONS.
THE INFUSION BAG AND PORTS ARE NOT MADE WITH NATURAL RUBBER LATEX.
Manufactured in UK for:
Mylan Institutional LLC
Morgantown, WV 26505 U.S.A.
Rx only
Mylan
| MAGNESIUM SULFATE IN DEXTROSE
magnesium sulfate heptahydrate injection, solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Mylan Institutional LLC (790384502) |