DABUR by Dabur India Limited White Petrolatum
DABUR by
Drug Labeling and Warnings
DABUR by is a Otc medication manufactured, distributed, or labeled by Dabur India Limited. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
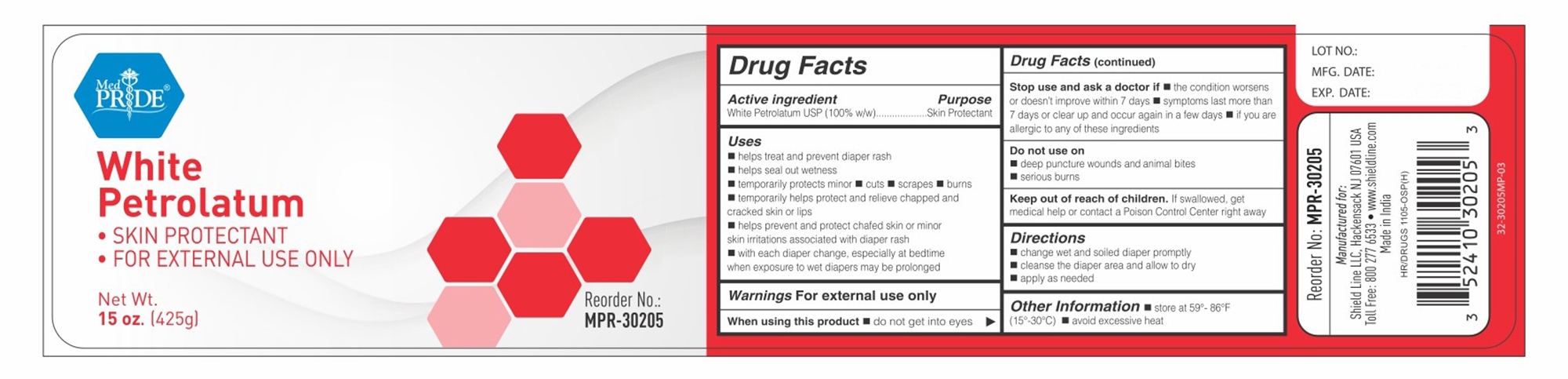
DABUR- white petrolatum ointment
Dabur India Limited
----------
White Petrolatum
Uses
■ helps treat and prevent diaper rash ■ helps seal out wetness ■ temporarily protects minor ■ cuts ■ scrapes ■ burns ■ temporarily helps protect and relieve chapped and cracked skin or lips ■ helps prevent and protect chafed skin or minor skin irritations associated with diaper rash ■ with each diaper change, espicially at bedtime when exposure to wet diapers may be prolonged
Stop use and ask a doctor if
■ condition worsens ■ symptoms last more than 7 days or clear up and occur again in a few days
Keep out of reach of children.If swallowed, get medical help or contact a Poison Control Center right away
| DABUR
white petrolatum ointment |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Dabur India Limited (650319218) |
Trademark Results [DABUR]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 DABUR 87935532 5607230 Live/Registered |
Dabur India Limited 2018-05-24 |
 DABUR 85825122 4388083 Live/Registered |
Dabur India Limited 2013-01-16 |
 DABUR 85825097 4395556 Live/Registered |
Dabur India Limited 2013-01-16 |
 DABUR 77342025 3530167 Live/Registered |
Dabur India Limited 2007-12-02 |
 DABUR 77122778 3529735 Live/Registered |
Dabur India Limited 2007-03-05 |
 DABUR 75507156 2628528 Live/Registered |
DABUR INDIA LIMITED 1998-06-23 |
 DABUR 74275280 1890384 Live/Registered |
DABUR INDIA LIMITED 1992-05-14 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.