MIRCERA- methoxy polyethylene glycol-epoetin beta injection, solution
Mircera by
Drug Labeling and Warnings
Mircera by is a Prescription medication manufactured, distributed, or labeled by Vifor (International) Inc., Roche Diagnostics GmbH, F. Hoffmann-La Roche Ltd.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use MIRCERA safely and effectively. See full prescribing information for MIRCERA.
MIRCERA® (methoxy polyethylene glycol-epoetin beta) injection, for intravenous or subcutaneous use
Initial U.S. Approval: 2007WARNING: ESAs INCREASE THE RISK OF DEATH, MYOCARDIAL INFARCTION, STROKE, VENOUS THROMBOEMBOLISM, THROMBOSIS OF VASCULAR ACCESS and TUMOR PROGRESSION OR RECURRENCE
See full prescribing information for complete boxed warning
Chronic Kidney Disease:
- In controlled trials, patients experienced greater risks for death, serious adverse cardiovascular reactions, and stroke when administered erythropoiesis-stimulating agents (ESAs) to target a hemoglobin level of greater than 11 g/dL (5.1).
- No trial has identified a hemoglobin target level, ESA dose, or dosing strategy that does not increase these risks (5.1).
- Use the lowest Mircera dose sufficient to reduce the need for red blood cell (RBC) transfusions (5.1).
Cancer:
- Mircera is not indicated and is not recommended for the treatment of anemia due to cancer chemotherapy. A dose-ranging study of Mircera was terminated early because of more deaths among patients receiving Mircera than another ESA (5.2).
- ESAs shortened overall survival and/or increased the risk of tumor progression or recurrence in clinical studies in patients with breast, non-small cell lung, head and neck, lymphoid, and cervical cancers (5.2).
RECENT MAJOR CHANGES
INDICATIONS AND USAGE
Mircera is an erythropoiesis-stimulating agent (ESA) indicated for the treatment of anemia associated with chronic kidney disease (CKD) in:
- adult patients on dialysis and adult patients not on dialysis (1.1).
- pediatric patients 5 to 17 years of age on hemodialysis who are converting from another ESA after their hemoglobin level was stabilized with an ESA (1.1).
Limitations of Use
Mircera is not indicated and is not recommended for use:
- In the treatment of anemia due to cancer chemotherapy (5.2).
- As a substitute for RBC transfusions in patients who require immediate correction of anemia (12.2).
Mircera has not been shown to improve quality of life, fatigue, or patient well-being.
DOSAGE AND ADMINISTRATION
In adult patients, Mircera is administered by subcutaneous or intravenous injection (2.2).
- Initial Treatment: 0.6 mcg/kg body weight administered once every two weeks (2.2).
- Conversion from Another ESA: dosed once monthly or once every two weeks based on total weekly epoetin alfa or darbepoetin alfa dose at time of conversion (2.2).
In pediatric patients, Mircera is administered by intravenous injection only (2.2).
- Conversion from Another ESA: dosed once every 4 weeks based on total weekly epoetin alfa or darbepoetin alfa dose at time of conversion (2.2).
DOSAGE FORMS AND STRENGTHS
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Hypertension: Control hypertension prior to initiating and during treatment with Mircera (5.3).
- Seizures: Seizures have occurred in CKD patients participating in Mircera clinical studies. Increase monitoring of these patients for changes in seizure frequency or premonitory symptoms (5.4).
- PRCA: If severe anemia and low reticulocyte count develop during Mircera treatment, withhold Mircera and evaluate for PRCA (5.6).
- Serious Allergic Reactions: Discontinue Mircera and manage reactions (5.7).
- Severe Cutaneous Reactions: Discontinue Mircera (5.8).
ADVERSE REACTIONS
The most common adverse reactions (≥ 10%) are hypertension, diarrhea, nasopharyngitis (6).
To report SUSPECTED ADVERSE REACTIONS, contact Vifor (International) Inc. at 1-800-576-8295, or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 6/2018
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: ESAs INCREASE THE RISK OF DEATH, MYOCARDIAL INFARCTION, STROKE, VENOUS THROMBOEMBOLISM, THROMBOSIS OF VASCULAR ACCESS and TUMOR PROGRESSION OR RECURRENCE
1 INDICATIONS AND USAGE
1.1 Anemia Due to Chronic Kidney Disease
2 DOSAGE AND ADMINISTRATION
2.1 Important Dosing Information
2.2 Patients with Chronic Kidney Disease
2.3 Preparation and Administration of Mircera
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Increased Mortality, Myocardial Infarction, Stroke, and Thromboembolism
5.2 Increased Mortality and/or Increased Risk of Tumor Progression or Recurrence in Patients with Cancer
5.3 Hypertension
5.4 Seizures
5.5 Lack or Loss of Hemoglobin Response to Mircera
5.6 Pure Red Cell Aplasia
5.7 Serious Allergic Reactions
5.8 Severe Cutaneous Reactions
5.9 Dialysis Management
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Immunogenicity
6.3 Postmarketing Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Adult Patients
14.2 Pediatric Patients on Hemodialysis
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: ESAs INCREASE THE RISK OF DEATH, MYOCARDIAL INFARCTION, STROKE, VENOUS THROMBOEMBOLISM, THROMBOSIS OF VASCULAR ACCESS and TUMOR PROGRESSION OR RECURRENCE
Chronic Kidney Disease [see Warnings and Precautions (5.1)]
- In controlled trials, patients experienced greater risks for death, serious adverse cardiovascular reactions, and stroke when administered erythropoiesis-stimulating agents (ESAs) to target a hemoglobin level of greater than 11 g/dL.
- No trial has identified a hemoglobin target level, ESA dose, or dosing strategy that does not increase these risks.
- Use the lowest Mircera dose sufficient to reduce the need for red blood cell (RBC) transfusions.
Cancer [see Warnings and Precautions (5.2)]
- Mircera is not indicated and is not recommended for the treatment of anemia due to cancer chemotherapy. A dose-ranging study of Mircera was terminated early because of more deaths among patients receiving Mircera than another ESA.
- ESAs have shown shortened overall survival and/or increased the risk of tumor progression or recurrence in clinical studies in patients with breast, non-small cell lung, head and neck, lymphoid, and cervical cancers.
-
1 INDICATIONS AND USAGE
1.1 Anemia Due to Chronic Kidney Disease
Mircera is indicated for the treatment of anemia associated with chronic kidney disease (CKD) in:
adult patients on dialysis and adult patients not on dialysis.
pediatric patients 5 to 17 years of age on hemodialysis who are converting from another ESA after their hemoglobin level was stabilized with an ESA.
Limitations of Use
Mircera is not indicated and is not recommended:
- In the treatment of anemia due to cancer chemotherapy [see Warnings and Precautions (5.2)].
- As a substitute for RBC transfusions in patients who require immediate correction of anemia [see Clinical Pharmacology (12.2)].
Mircera has not been shown to improve symptoms, physical functioning, or health-related quality of life.
-
2 DOSAGE AND ADMINISTRATION
2.1 Important Dosing Information
Evaluation of Iron Stores and Nutritional Factors
Evaluate the iron status in all patients before and during treatment. Administer supplemental iron therapy when serum ferritin is less than 100 mcg/L or when serum transferrin saturation is less than 20%. The majority of patients with CKD will require supplemental iron during the course of ESA therapy.
Monitoring of Response to Therapy
Correct or exclude other causes of anemia (e.g., vitamin deficiency, metabolic or chronic inflammatory conditions, bleeding, etc.) before initiating Mircera [see Warnings and Precautions (5.9)]. Following initiation of therapy and after each dose adjustment, monitor hemoglobin weekly until the hemoglobin level is stable and sufficient to minimize the need for RBC transfusion.
2.2 Patients with Chronic Kidney Disease
Individualize dosing and use the lowest dose of Mircera sufficient to reduce the need for RBC transfusions [see Warnings and Precautions (5.1)]. In controlled trials, patients experienced greater risks for death, serious adverse cardiovascular reactions, and stroke when administered erythropoiesis-stimulating agents (ESAs) to target a hemoglobin level of greater than 11 g/dL. No trial has identified a hemoglobin target level, ESA dose, or dosing strategy that does not increase these risks. Physicians and patients should weigh the possible benefits of decreasing transfusions against the increased risks of death and other serious cardiovascular adverse events [see Boxed Warning and Clinical Studies (14)].
For all patients with CKD:
When initiating or adjusting therapy, monitor hemoglobin levels at least weekly until stable, then monitor at least monthly. When adjusting therapy consider hemoglobin rate of rise, rate of decline, ESA responsiveness and hemoglobin variability. A single hemoglobin excursion may not require a dosing change.
- Do not increase the dose more frequently than once every 4 weeks. Decreases in dose can occur more frequently. Avoid frequent dose adjustments.
- If the hemoglobin rises rapidly (e.g., more than 1 g/dL in any 2-week period), reduce the dose of Mircera by 25% or more as needed to reduce rapid responses.
- For patients who do not respond adequately, if the hemoglobin has not increased by more than 1 g/dL after 4 weeks of therapy, increase the dose by 25%.
- For patients who do not respond adequately over a 12-week escalation period, increasing the Mircera dose further is unlikely to improve response and may increase risks. Use the lowest dose that will maintain a hemoglobin level sufficient to reduce the need for RBC transfusions. Evaluate other causes of anemia. Discontinue Mircera if responsiveness does not improve.
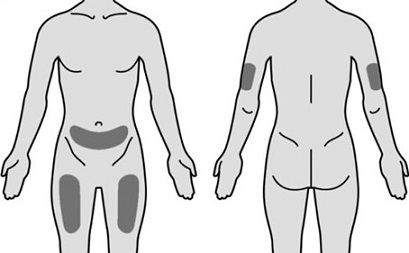
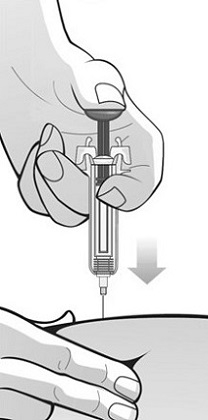
Administer Mircera either intravenously or subcutaneously in adult patients and only intravenously in pediatric patients. When administered subcutaneously, Mircera should be injected in the abdomen, arm or thigh.
For Adult Patients with CKD on dialysis:
- Initiate Mircera treatment when the hemoglobin level is less than 10 g/dL.
- If the hemoglobin level approaches or exceeds 11 g/dL, reduce or interrupt the dose of Mircera.
- The recommended starting dose of Mircera for the treatment of anemia in adult CKD patients who are not currently treated with an ESA is 0.6 mcg/kg body weight administered as a single intravenous or subcutaneous injection once every two weeks. The intravenous route is recommended for patients receiving hemodialysis because the intravenous route may be less immunogenic [see Adverse Reactions (6.2)].
- Once the hemoglobin has been stabilized, Mircera may be administered once monthly using a dose that is twice that of the every-two-week dose and subsequently titrated as necessary.
For Adult Patients with CKD not on dialysis:
-
Consider initiating Mircera treatment only when the hemoglobin level is less than 10 g/dL and the following considerations apply:
- o The rate of hemoglobin decline indicates the likelihood of requiring a RBC transfusion and,
- o Reducing the risk of alloimmunization and/or other RBC transfusion-related risks is a goal.
- If the hemoglobin level exceeds 10 g/dL, reduce or interrupt the dose of Mircera, and use the lowest dose of Mircera sufficient to reduce the need for RBC transfusions.
- The recommended starting dose of Mircera for the treatment of anemia in adult CKD patients who are not currently treated with an ESA is 0.6 mcg/kg body weight administered as a single intravenous or subcutaneous injection once every two weeks.
- Once the hemoglobin has been stabilized, Mircera may be administered once monthly using a dose that is twice that of the every-two-week dose and subsequently titrated as necessary.
Refer patients who self-administer Mircera to the Instructions for Use [see Patient Counseling Information (17)].
Conversion from Epoetin alfa or Darbepoetin alfa to Mircera in Adult Patients with CKD
Mircera can be administered once every two weeks or once monthly to patients whose hemoglobin has been stabilized by treatment with an ESA (see Table 1). The dose of Mircera, given as a single intravenous or subcutaneous injection, should be based on the total weekly ESA dose at the time of conversion.
Table 1 Mircera Starting Doses for Adult Patients Currently Receiving an ESA Previous Weekly
Epoetin alfa
Dose
(units/week)
Previous Weekly
Darbepoetin alfa Dose
(mcg/week)
Mircera Dose
Once Monthly (mcg/month)
Once Every Two Weeks (mcg/every two weeks)
less than 8000
less than 40
120
60
8000 to 16000
40 to 80
200
100
more than 16000
more than 80
360
180
For Pediatric Patients with CKD on hemodialysis:
Conversion from Epoetin alfa or Darbepoetin alfa to Mircera in Pediatric Patients with CKD Treated with Hemodialysis
Administer Mircera intravenously once every 4 weeks to pediatric patients (ages 5 to 17 years) whose hemoglobin level has been stabilized by treatment with an ESA. Administer Mircera as an intravenous injection at the dose (in micrograms) based on the total weekly ESA dose at the time of conversion (see Table 2).
Table 2 Mircera Starting Doses for Pediatric Patients Currently Receiving an ESA Epoetin alfa
Darbepoetin alfa
4 x previous weekly epoetin alfa dose (Units)/125
e.g., 4 x 1500 Units of epoetin alfa per week/125 = 48 mcg of Mircera once every 4 weeks
4 x previous weekly darbepoetin alfa dose (mcg)/0.55
e.g., 4 x 20 mcg of darbepoetin alfa per week/0.55 = 145.5 mcg of Mircera once every 4 weeks
2.3 Preparation and Administration of Mircera
Mircera is packaged as single-dose prefilled syringes. Mircera contains no preservatives. Discard any unused portion. Do not pool unused portions from the prefilled syringes. Do not use the prefilled syringe more than once.
Always store Mircera prefilled syringes in their original cartons. Vigorous shaking or prolonged exposure to light should be avoided.
Do not mix Mircera with any parenteral solution.
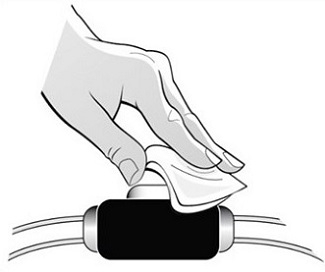
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration. Do not use any prefilled syringes exhibiting particulate matter or a coloration other than colorless to slightly yellowish.
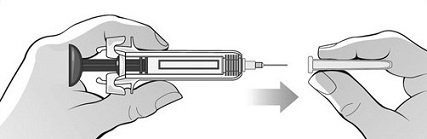
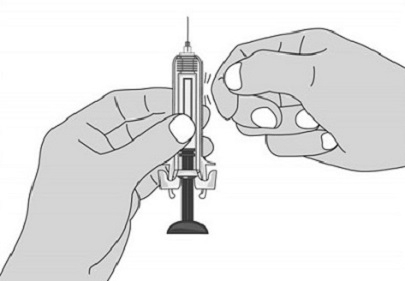
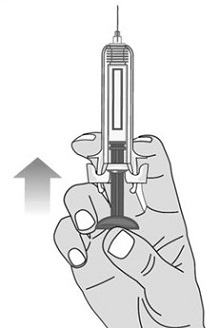
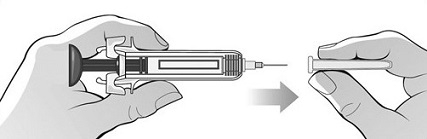
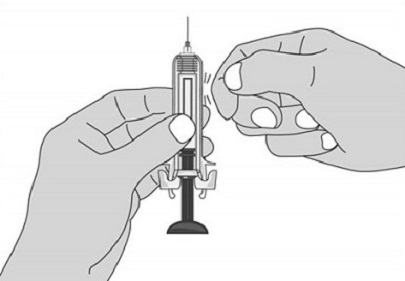
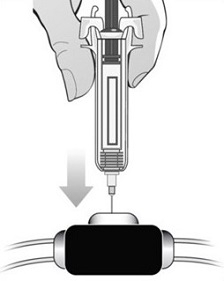
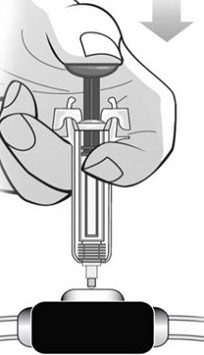
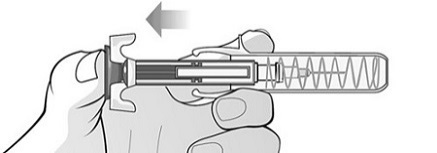
For administration using the prefilled syringe, the plunger must be fully depressed during injection in order for the needle guard to activate. Following administration, remove the needle from the injection site and then release the plunger to allow the needle guard to move up until the entire needle is covered.
See “Instructions for Use” for complete instructions on the preparation and administration of Mircera. Examine each prefilled syringe for the expiration date. Do not use Mircera after the expiration date.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
Mircera is contraindicated in patients with:
- Uncontrolled hypertension [see Warnings and Precautions (5.3)]
- Pure red cell aplasia (PRCA) that begins after treatment with Mircera or other erythropoietin protein drugs [see Warnings and Precautions (5.6)]
- History of serious or severe allergic reactions to Mircera (e.g. anaphylactic reactions, angioedema, bronchospasm, skin rash, and urticaria) [see Warnings and Precautions (5.7, 5.8)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Increased Mortality, Myocardial Infarction, Stroke, and Thromboembolism
- In controlled clinical trials of patients with CKD comparing higher hemoglobin targets (13 to 14 g/dL) to lower targets (9 to 11.3 g/dL), ESAs increased the risk of death, myocardial infarction, stroke, congestive heart failure, thrombosis of hemodialysis vascular access, and other thromboembolic events in the higher target groups.
- Using ESAs to target a hemoglobin level of greater than 11 g/dL increases the risk of serious adverse cardiovascular reactions and has not been shown to provide additional benefit [see Clinical Studies (14)]. Use caution in patients with coexistent cardiovascular disease and stroke [see Dosage and Administration (2.2)]. Patients with CKD and an insufficient hemoglobin response to ESA therapy may be at even greater risk for cardiovascular reactions and mortality than other patients. A rate of hemoglobin rise of greater than 1 g/dL over 2 weeks may contribute to these risks.
- In controlled clinical trials of patients with cancer, ESAs increased the risks for death and serious adverse cardiovascular reactions. These adverse reactions included myocardial infarction and stroke.
- In controlled clinical trials, ESAs increased the risk of death in patients undergoing coronary artery bypass graft surgery (CABG) and the risk of deep venous thrombosis (DVT) in patients undergoing orthopedic procedures.
The design and overall results of the 3 large trials comparing higher and lower hemoglobin targets are shown in Table 3 (Normal Hematocrit Study (NHS), Correction of Hemoglobin Outcomes in Renal Insufficiency (CHOIR) and Trial to Reduce Cardiovascular Events with Aranesp® Therapy (TREAT)).
Table 3 Randomized Controlled Trials Showing Adverse Cardiovascular Outcomes in Patients With CKD NHS
(N = 1265)CHOIR
(N = 1432)TREAT
(N = 4038)Time Period of Trial
1993 to 1996
2003 to 2006
2004 to 2009
Population
CKD patients on hemodialysis with coexisting CHF or CAD, hematocrit 30 ± 3% on epoetin alfa
CKD patients not on dialysis with hemoglobin less than 11 g/dL not previously administered epoetin alfa
CKD patients not on dialysis with type II diabetes, hemoglobin ≤ 11 g/dL
Hemoglobin Target;
Higher vs. Lower (g/dL)14.0 vs. 10.0
13.5 vs. 11.3
13.0 vs. ≥ 9.0
Median (Q1, Q3)
Achieved Hemoglobin level (g/dL)12.6 (11.6, 13.3) vs.
10.3 (10.0, 10.7)13.0 (12.2, 13.4) vs.
11.4 (11.1, 11.6)12.5 (12.0, 12.8) vs.
10.6 (9.9, 11.3)Primary Endpoint
All-cause mortality
or nonfatal MIAll-cause mortality,
MI, hospitalization for CHF, or strokeAll-cause mortality,
MI, myocardial ischemia, heart failure, and strokeHazard Ratio or Relative Risk (95% CI)
1.28 (1.06 to 1.56)
1.34 (1.03 to 1.74)
1.05 (0.94 to 1.17)
Adverse Outcome for Higher Target Group
All-cause mortality
All-cause mortality
Stroke
Hazard Ratio or Relative Risk (95% CI)
1.27 (1.04 to 1.54)
1.48 (0.97 to 2.27)
1.92 (1.38 to 2.68)
Patients with Chronic Kidney Disease
NHS: A prospective, randomized, open-label study of 1265 patients with chronic kidney disease on dialysis with documented evidence of congestive heart failure or ischemic heart disease was designed to test the hypothesis that a higher target hematocrit (Hct) would result in improved outcomes compared with a lower target Hct. In this study, patients were randomized to epoetin alfa treatment targeted to a maintenance hemoglobin of either 14 ± 1 g/dL or 10 ± 1 g/dL. The trial was terminated early with adverse safety findings of higher mortality in the high hematocrit target group. Higher mortality (35% vs. 29%) was observed for the patients randomized to a target hemoglobin of 14 g/dL than for the patients randomized to a target hemoglobin of 10 g/dL. For all-cause mortality, the HR=1.27; 95% CI (1.04, 1.54); p=0.018. The incidence of nonfatal myocardial infarction, vascular access thrombosis, and other thrombotic events was also higher in the group randomized to a target hemoglobin of 14 g/dL.
CHOIR: In a randomized prospective trial, 1432 patients with anemia due to CKD who were not undergoing dialysis were assigned to epoetin alfa treatment targeting a maintenance hemoglobin concentration of 13.5 g/dL or 11.3 g/dL. The trial was terminated early with adverse safety findings. A major cardiovascular event (death, myocardial infarction, stroke, or hospitalization for congestive heart failure) occurred among 125 (18%) of the 715 patients in the higher hemoglobin group compared to 97 (14%) among the 717 patients in the lower hemoglobin group (HR 1.3, 95% CI: 1.0, 1.7 p=0.03).
TREAT: A randomized, double-blind, placebo-controlled, prospective trial of 4038 patients with: CKD not on dialysis (eGFR of 20 – 60 mL/min), anemia (hemoglobin levels ≤ 11 g/dL), and type 2 diabetes mellitus, patients were randomized to receive either darbepoetin alfa treatment or a matching placebo. Placebo group patients also received darbepoetin alfa when their hemoglobin levels were below 9 g/dL. The trial objectives were to demonstrate the benefit of darbepoetin alfa treatment of the anemia to a target hemoglobin level of 13 g/dL, when compared to a "placebo" group, by reducing the occurrence of either of two primary endpoints: (1) a composite cardiovascular endpoint of all-cause mortality or a specified cardiovascular event (myocardial ischemia, CHF, MI, and CVA) or (2) a composite renal endpoint of all-cause mortality or progression to end stage renal disease. The overall risks for each of the two primary endpoints (the cardiovascular composite and the renal composite) were not reduced with darbepoetin alfa treatment (see Table 3), but the risk of stroke was increased nearly two-fold in the darbepoetin alfa-treated group versus the placebo group: annualized stroke rate 2.1% vs. 1.1%, respectively, HR 1.92; 95% CI: 1.38, 2.68; p less than 0.001. The relative risk of stroke was particularly high in patients with a prior stroke: annualized stroke rate 5.2% in the darbepoetin alfa-treated group and 1.9% in the placebo group, HR 3.07; 95% CI: 1.44, 6.54. Also, among darbepoetin alfa-treated subjects with a past history of cancer, there were more deaths due to all causes and more deaths adjudicated as due to cancer, in comparison with the control group.
Patients with Cancer
An increased incidence of thromboembolic reactions, some serious and life-threatening, occurred in patients with cancer treated with ESAs.
In a randomized, placebo-controlled study (Study 1 in Table 4 [see Warnings and Precautions (5.2)]) of 939 women with metastatic breast cancer receiving chemotherapy, patients received either weekly epoetin alfa or placebo for up to a year. This study was designed to show that survival was superior when epoetin alfa was administered to prevent anemia (maintain hemoglobin levels between 12 and 14 g/dL or hematocrit between 36% and 42%). This study was terminated prematurely when interim results demonstrated a higher mortality at 4 months (8.7% vs. 3.4%) and a higher rate of fatal thrombotic reactions (1.1% vs. 0.2%) in the first 4 months of the study among patients treated with epoetin alfa. Based on Kaplan-Meier estimates, at the time of study termination, the 12-month survival was lower in the epoetin alfa group than in the placebo group (70% vs. 76%; HR 1.37, 95% CI: 1.07, 1.75; p = 0.012).
Patients Having Surgery
Mircera is not approved for reduction of RBC transfusions in patients scheduled for surgical procedures.
An increased incidence of deep vein thrombosis (DVT) in patients receiving epoetin alfa undergoing surgical orthopedic procedures has been observed. In a randomized controlled study (referred to as the “SPINE” study), 681 adult patients, not receiving prophylactic anticoagulation and undergoing spinal surgery, received epoetin alfa and standard of care (SOC) treatment, or SOC treatment alone. Preliminary analysis showed a higher incidence of DVT, determined by either Color Flow Duplex Imaging or by clinical symptoms, in the epoetin alfa group [16 patients (4.7%)] compared to the SOC group [7 patients (2.1%)]. In addition, 12 patients in the epoetin alfa group and 7 patients in the SOC group had other thrombotic vascular events.
Increased mortality was observed in a randomized placebo-controlled study of epoetin alfa in adult patients who were undergoing coronary artery bypass surgery (7 deaths in 126 patients randomized to epoetin alfa versus no deaths among 56 patients receiving placebo). Four of these deaths occurred during the period of study drug administration and all four deaths were associated with thrombotic events.
5.2 Increased Mortality and/or Increased Risk of Tumor Progression or Recurrence in Patients with Cancer
Mircera is not indicated and is not recommended for use in the treatment of anemia due to cancer chemotherapy. A dose-ranging trial of Mircera in 153 patients who were undergoing chemotherapy for non-small cell lung cancer was terminated prematurely because more deaths occurred among patients receiving Mircera than another ESA.
ESAs resulted in decreased locoregional control/progression-free survival and/or overall survival (see Table 4). These findings were observed in studies of patients with advanced head and neck cancer receiving radiation therapy (Studies 5 and 6), in patients receiving chemotherapy for metastatic breast cancer (Study 1) or lymphoid malignancy (Study 2), and in patients with non-small cell lung cancer or various malignancies who were not receiving chemotherapy or radiotherapy (Studies 7 and 8).
Table 4 Randomized, Controlled Trials with Decreased Survival and/or Decreased Locoregional Control - * Q1= 25th percentile; Q3= 75th percentile
Study/Tumor
(n)Hemoglobin
TargetAchieved
Hemoglobin
(Median
Q1,Q3*)Primary Endpoint
Adverse Outcome for ESA-containing Arm
Chemotherapy
Cancer Study 1
Metastatic breast
cancer
(n=939)12 to 14 g/dL
12.9 g/dL
12.2, 13.3 g/dL12-month overall
survivalDecreased 12-month survival
Cancer Study 2
Lymphoid malignancy
(n=344)13 to 15 g/dL (M)
13 to 14 g/dL (F)11.0 g/dL
9.8, 12.1 g/dLProportion of patients achieving a hemoglobin response
Decreased overall survival
Cancer Study 3
Early breast cancer
(n=733)12.5 to 13 g/dL
13.1 g/dL
12.5, 13.7 g/dLRelapse-free and overall survival
Decreased 3-year relapse-free and overall survival
Cancer Study 4
Cervical cancer
(n=114)12 to 14 g/dL
12.7 g/dL
12.1, 13.3 g/dLProgression-free and overall survival and locoregional control
Decreased 3-year progression-free and overall survival and locoregional control
Radiotherapy Alone
Cancer Study 5
Head and neck
cancer
(n=351)more than 15 g/dL (M)
more than 14 g/dL (F)Not available
Locoregional progression-free survival (LRPFS)
Decreased 5-year locoregional progression-free survival
Decreased overall survivalCancer Study 6
Head and neck
cancer
(n=522)14 to 15.5 g/dL
Not available
Locoregional disease control (LRC)
Decreased locoregional disease control
No Chemotherapy or Radiotherapy
Cancer Study 7
Non-small cell lung cancer
(n=70)12 to 14 g/dL
Not available
Quality of life
Decreased overall survival
Cancer Study 8
Non-myeloid malignancy
(n=989)12 to 13 g/dL
10.6 g/dL
9.4, 11.8 g/dLRBC transfusions
Decreased overall survival
Decreased Overall Survival:
Cancer Study 1 (the “BEST” study) was previously described [see Warnings and Precautions (5.1)]. Mortality at 4 months (8.7% vs. 3.4%) was significantly higher in the epoetin alfa arm. The most common investigator-attributed cause of death within the first 4 months was disease progression; 28 of 41 deaths in the epoetin alfa arm and 13 of 16 deaths in the placebo arm were attributed to disease progression. Investigator assessed time to tumor progression was not different between the two groups. Survival at 12 months was significantly lower in the epoetin alfa arm (70% vs. 76%, HR 1.37, 95% CI: 1.07, 1.75; p=0.012).
Cancer Study 2 was a Phase 3, double-blind, randomized (darbepoetin alfa vs. placebo) study conducted in 344 anemic patients with lymphoid malignancy receiving chemotherapy. With a median follow-up of 29 months, overall mortality rates were significantly higher among patients randomized to darbepoetin alfa as compared to placebo (HR 1.36, 95% CI: 1.02, 1.82).
Cancer Study 7 was a Phase 3, multicenter, randomized (epoetin alfa vs. placebo), double-blind study, in which patients with advanced non-small cell lung cancer receiving only palliative radiotherapy or no active therapy were treated with epoetin alfa to achieve and maintain hemoglobin levels between 12 and 14 g/dL. Following an interim analysis of 70 of 300 patients planned, a significant difference in survival in favor of the patients on the placebo arm of the trial was observed (median survival 63 vs. 129 days; HR 1.84; p=0.04).
Cancer Study 8 was a Phase 3, double-blind, randomized (darbepoetin alfa vs. placebo), 16-week study in 989 anemic patients with active malignant disease, neither receiving nor planning to receive chemotherapy or radiation therapy. There was no evidence of a statistically significant reduction in proportion of patients receiving RBC transfusions. The median survival was shorter in the darbepoetin alfa treatment group (8 months) compared with the placebo group (10.8 months); HR 1.30, 95% CI: 1.07, 1.57.
Decreased Progression-Free Survival and Overall Survival:
Cancer Study 3 (the “PREPARE” study) was a randomized controlled study in which darbepoetin alfa was administered to prevent anemia conducted in 733 women receiving neo-adjuvant breast cancer treatment. A final analysis was performed after a median follow-up of approximately 3 years at which time the survival rate was lower (86% vs. 90%, HR 1.42, 95% CI: 0.93, 2.18) and relapse-free survival rate was lower (72% vs. 78%, HR 1.33, 95% CI: 0.99, 1.79) in the darbepoetin alfa-treated arm compared to the control arm.
Cancer Study 4 (protocol GOG 191) was a randomized controlled study that enrolled 114 of a planned 460 cervical cancer patients receiving chemotherapy and radiotherapy. Patients were randomized to receive epoetin alfa to maintain hemoglobin between 12 and 14 g/dL or to transfusion support as needed. The study was terminated prematurely due to an increase in thromboembolic events in epoetin alfa-treated patients compared to control (19% vs. 9%). Both local recurrence (21% vs. 20%) and distant recurrence (12% vs. 7%) were more frequent in epoetin alfa-treated patients compared to control. Progression-free survival at 3 years was lower in the epoetin alfa-treated group compared to control (59% vs. 62%, HR 1.06, 95% CI: 0.58, 1.91). Overall survival at 3 years was lower in the epoetin alfa-treated group compared to control (61% vs. 71%, HR 1.28, 95% CI: 0.68, 2.42).
Cancer Study 5 (the “ENHANCE” study) was a randomized controlled study in 351 head and neck cancer patients where epoetin beta or placebo was administered to achieve target hemoglobins of 14 and 15 g/dL for women and men, respectively. Locoregional progression-free survival was significantly shorter in patients receiving epoetin beta (HR 1.62, 95% CI: 1.22, 2.14, p=0.0008) with a median of 406 days epoetin beta vs. 745 days placebo. Overall survival was significantly shorter in patients receiving epoetin beta (HR 1.39, 95% CI: 1.05, 1.84; p=0.02).
Decreased Locoregional Control:
Cancer Study 6 (DAHANCA 10) was conducted in 522 patients with primary squamous cell carcinoma of the head and neck receiving radiation therapy randomized to darbepoetin alfa with radiotherapy or radiotherapy alone. An interim analysis on 484 patients demonstrated that locoregional control at 5 years was significantly shorter in patients receiving darbepoetin alfa (RR 1.44, 95% CI: 1.06, 1.96; p=0.02). Overall survival was shorter in patients receiving darbepoetin alfa (RR 1.28, 95% CI: 0.98, 1.68; p=0.08).
5.3 Hypertension
Mircera is contraindicated in patients with uncontrolled hypertension.
In Mircera clinical studies, approximately 27% of patients with CKD, including patients on dialysis and patients not on dialysis, required intensification of antihypertensive therapy. Hypertensive encephalopathy and/or seizures have been observed in patients with CKD treated with Mircera [see Warnings and Precautions (5.4)].
Appropriately control hypertension prior to initiation of and during treatment with Mircera. Reduce or withhold Mircera if blood pressure becomes difficult to control. Advise patients of the importance of compliance with antihypertensive therapy and dietary restrictions [see Patient Counseling Information (17)].
5.4 Seizures
Seizures have occurred in patients participating in Mircera clinical studies. During the first several months following initiation of Mircera, monitor patients closely for premonitory neurologic symptoms. Advise patients to contact their healthcare practitioner for new-onset seizures, premonitory symptoms, or change in seizure frequency.
5.5 Lack or Loss of Hemoglobin Response to Mircera
For lack or loss of hemoglobin response to Mircera, initiate a search for causative factors (e.g., iron deficiency, infection, inflammation, bleeding).
If typical causes of lack or loss of hemoglobin response are excluded, evaluate for PRCA [see Warnings and Precautions (5.6)]. In the absence of PRCA, follow dosing recommendations for management of patients with an insufficient response to Mircera therapy [see Dosage and Administration (2.2)].
5.6 Pure Red Cell Aplasia
Cases of PRCA and of severe anemia, with or without other cytopenias that arise following the development of neutralizing antibodies to erythropoietin have been reported in the postmarketing setting in patients treated with Mircera. This has been reported predominantly in patients with CKD receiving ESAs by subcutaneous administration. PRCA was not observed in clinical studies of Mircera.
PRCA has also been reported in patients receiving ESAs for anemia related to hepatitis C treatment (an indication for which Mircera is not approved).
If severe anemia and low reticulocyte count develop during treatment with Mircera, withhold Mircera and evaluate patients for neutralizing antibodies to erythropoietin [see Warnings and Precautions (5.5)]. Serum samples should be obtained at least a month after the last Mircera administration to prevent interference of Mircera with the assay. Contact Vifor at 1-800-576-8295 to perform assays for binding and neutralizing antibodies. Permanently discontinue Mircera in patients who develop PRCA following treatment with Mircera or other erythropoietin protein drugs. Do not switch patients to other ESAs as antibodies may cross-react [see Adverse Reactions (6.2)].
5.7 Serious Allergic Reactions
Serious allergic reactions, including anaphylactic reactions, angioedema, bronchospasm, tachycardia, pruritus skin rash and urticaria have been reported in patients treated with Mircera. If a serious allergic or anaphylactic reaction occurs due to Mircera, immediately and permanently discontinue Mircera and administer appropriate therapy.
5.8 Severe Cutaneous Reactions
Blistering and skin exfoliation reactions including Erythema multiforme and Stevens-Johnson Syndrome (SJS)/Toxic Epidermal Necrolysis (TEN), have been reported in patients treated with ESAs (including Mircera) in the postmarketing setting. Discontinue Mircera therapy immediately if a severe cutaneous reaction, such as SJS/TEN, is suspected.
-
6 ADVERSE REACTIONS
The following serious adverse reactions are discussed in greater detail in other sections of the labeling:
- Increased Mortality, Myocardial Infarction, Stroke, and Thromboembolism [see Warnings and Precautions (5.1)]
- Increased Mortality and/or Tumor Progression in Patients with Cancer [see Warnings and Precautions (5.2)]
- Hypertension [see Warnings and Precautions (5.3)]
- Seizures [see Warnings and Precautions (5.4)]
- Pure Red Cell Aplasia [see Warnings and Precautions (5.6)]
- Serious Allergic Reactions [see Warnings and Precautions (5.7)]
- Severe Cutaneous Reactions [see Warnings and Precautions (5.8)]
6.1 Clinical Trials Experience
Adult Patients
The data described below reflect exposure to Mircera in 2737 patients, including 1451 exposed for 6 months and 1144 exposed for greater than one year. Mircera was studied primarily in active-controlled studies (n=1789 received Mircera, and n=948 received another ESA) and in long-term follow up studies. The population was 18 to 92 years of age, 58% male, and the percentage of Caucasian, Black (including African Americans), Asian and Hispanic patients were 73%, 20%, 5%, and 9%, respectively. Approximately 85% of the patients were receiving dialysis. Most patients received Mircera using dosing regimens of once every two or four weeks, administered subcutaneously or intravenously.
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of Mircera cannot be directly compared to rates in the clinical trials of other drugs and may not reflect the rates observed in practice.
The most commonly reported adverse reactions in ≥10% of patients were hypertension [see Warnings and Precautions (5.3)], diarrhea, and nasopharyngitis. The most common adverse reactions that led to treatment discontinuation in the Mircera clinical studies were: hypertension, coronary artery disease, anemia, concomitant termination of other CKD therapy and septic shock. Some of the adverse reactions reported are typically associated with CKD, or recognized complications of dialysis, and may not necessarily be attributable to Mircera therapy. Adverse reaction rates did not importantly differ between patients receiving Mircera or another ESA.
Table 5 summarizes the most frequent adverse reactions (≥ 5%) in patients treated with Mircera.
Table 5 Adverse Reactions Occurring in ≥ 5% of CKD Patients BODY SYSTEM Patients Treated with Mircera
(n=1789)Adverse Reaction VASCULAR
Hypertension
13%
Hypotension
5%
GASTROINTESTINAL
Diarrhea
11%
Vomiting
6%
Constipation
5%
INFECTIONS AND INFESTATIONS
Nasopharyngitis
11%
Upper Respiratory Tract Infection
9%
Urinary Tract Infection
5%
NERVOUS SYSTEM
Headache
9%
MUSCULOSKELETAL AND CONNECTIVE TISSUE
Muscle Spasms
8%
Back Pain
6%
Pain in Extremity
5%
INJURY, POISONING AND PROCEDURAL COMPLICATIONS
Procedural Hypotension
8%
Arteriovenous Fistula Thrombosis
5%
Arteriovenous Fistula Site Complication
5%
METABOLISM AND NUTRITION
Fluid Overload
7%
RESPIRATORY, THORACIC AND MEDIASTINAL
Cough
6%
In the controlled trials, the rates of serious adverse reactions did not importantly differ between patients receiving Mircera and another ESA (38% vs. 42%) except for the occurrence of serious gastrointestinal hemorrhage (1.2% vs. 0.2%). Serious hemorrhagic adverse reactions of all types occurred among 5% and 4% of patients receiving Mircera or another ESA, respectively.
Pediatric Patients
In an open-label, multiple dose study, 64 pediatric patients (ages 5 to 17 years) with CKD who were on hemodialysis and who had stable hemoglobin levels while previously receiving another ESA (epoetin alfa/beta or darbepoetin alfa) were then converted to Mircera administered intravenously once every 4 weeks for 20 weeks (core study period). Patients who completed the core study period with hemoglobin within ± 1 g/dL of their baseline hemoglobin and within the target range of 10 to 12 g/dL were eligible to enter an optional 52-week safety extension period (total duration of treatment, up to 73 weeks). In the extension period, 25 (out of 37) patients were treated for at least an additional 5 months. During the whole study (core study and safety extension), 33 patients were exposed to Mircera for at least 6 months and 19 were exposed for greater than 15 months.
All reported adverse reactions regardless of causality (more than 5% incidence) in the pediatric population included headache (22%), nasopharyngitis (22%), hypertension (19%), vomiting (11%), bronchitis (9%), abdominal pain (8%), arteriovenous fistula thrombosis (6%), cough (6%), device related infection (6%), hyperkalemia (6%), pharyngitis (6%), pyrexia (6%), thrombocytopenia (6%), and thrombosis in device (6%).
6.2 Immunogenicity
As with all therapeutic proteins, there is potential for immunogenicity. The detection of antibody formation is highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies to Mircera in the studies described below with the incidence of antibodies in other studies or to other products may be misleading.
Neutralizing antibodies to Mircera that cross-react with endogenous erythropoietin and other ESAs can result in PRCA or severe anemia (with or without other cytopenias) [see Warnings and Precautions (5.6)]. Compared to subcutaneous administration, the intravenous route of administration may lessen the risk for development of antibodies to Mircera.
In 1789 patients treated with Mircera in clinical studies, antibody testing using an enzyme-linked immunosorbent assay (ELISA) was conducted at baseline and during treatment. Antibody development was not detected in any of the patients.
6.3 Postmarketing Experience
The following adverse reactions have been identified during postapproval use of Mircera. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Hypersensitivity
Stevens-Johnson syndrome/toxic epidermal necrolysis has been reported [see Warnings and Precautions (5.7)].
Pure Red Cell Aplasia (PRCA)
Cases of PRCA and of severe anemia, with or without other cytopenias that arise following the development of neutralizing antibodies to erythropoietin have been reported in patients treated with Mircera [see Warnings and Precautions (5.6)].
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Available data from a small number of published case reports and postmarketing experience with Mircera use in pregnancy are insufficient to identify a drug associated risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes. Chronic kidney disease is associated with maternal and embryo-fetal risks (see Clinical Considerations). In animal reproduction studies, administration of methoxy polyethylene glycol-epoetin beta to rats and rabbits during pregnancy and lactation adversely affected offspring at doses 17-fold and greater than the recommended human dose (see Data).
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risks of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Clinical Considerations
Disease Associated Maternal and/or Embryo-Fetal Risk
Pregnancy in women with chronic kidney disease has been associated with adverse outcomes including hypertension, pre-eclampsia, miscarriage, premature birth, low-birth-weight, polyhydramnios, and intrauterine growth restriction.
Data
When methoxy polyethylene glycol-epoetin beta was administered subcutaneously to rats and rabbits during gestation (including the period of organogenesis), bone malformation was observed in both species at 50 mcg/kg once every three days (corresponding to 500 mcg/kg/month or 417-fold the recommended human dose) in studies of embryo-fetal development. This effect was observed as missing caudal vertebrae resulting in a thread-like tail in one rat fetus, absent first digit metacarpal and phalanx on each forelimb resulting in absent pollex in one rabbit fetus, and fused fourth and fifth cervical vertebrae centra in another rabbit fetus. Dose-related reduction in fetal weights was observed in both rats and rabbits. At doses 5 mcg/kg once every three days and higher, corresponding to 50 mcg/kg/month or 42-fold the recommended human dose, methoxy polyethylene glycol-epoetin beta caused exaggerated pharmacodynamic effects in dams.
Once-weekly doses of methoxy polyethylene glycol-epoetin beta up to 50 mcg/kg/dose (corresponding to 200 mcg/kg/month or 167-fold the recommended human dose) given to pregnant and lactating rats did not adversely affect pregnancy parameters, natural delivery or litter observations in a study of pre-and postnatal development. Increased deaths and significant reduction in the growth rate of the F1 generation were observed during lactation and early post weaning period at 20 and 50 mcg/kg/dose, corresponding to 80 and 200 mcg/kg/month or 67- and 167-fold the recommended human dose. A significant reduction in the growth rate of the F1 generation was evident already at 5 mcg/kg/dose, corresponding to 20 mcg/kg/month or 17-fold the recommended human dose. However, no remarkable effect on reflex, physical and cognitive development or reproductive performance was observed in F1 generation of any dose groups.
The dose level not causing any adverse effect on dams or offspring was not determined.
8.2 Lactation
Risk Summary
There are no data on the presence of methoxy polyethylene glycol-epoetin beta in human milk, the effects on the breastfed child, or the effects on milk production. However, endogenous erythropoietin is present in human milk. In rats, methoxy polyethylene glycol-epoetin beta was present in maternal milk (see Data). When a drug is present in animal milk, it is likely that the drug will be present in human milk. The lack of clinical data during lactation precludes a clear determination of the risk of Mircera to a child during lactation. Therefore, the developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for Mircera and any potential adverse effects on the breastfed child from Mircera or from the underlying maternal condition.
Data
A dose of methoxy polyethylene glycol-epoetin beta approximately 3-fold greater than the recommended human dose was administered to lactating rats. Methoxy polyethylene glycol-epoetin beta was detected in maternal milk 4 hours postdose and reached maximum concentration 48 hours postdose. The maximum amount of methoxy polyethylene glycol-epoetin beta in milk was about 10-fold lower than in serum. The concentration of drug in animal milk does not necessarily predict the concentration of drug in human milk.
8.4 Pediatric Use
The safety and effectiveness of Mircera for the treatment of anemia due to CKD have been established in pediatric patients 5 to 17 years of age on hemodialysis who are converting from another ESA after their hemoglobin level was stabilized with an ESA. The use of Mircera in this pediatric age group is supported by evidence from adequate and well-controlled studies of Mircera in adults and a dose-finding study in 64 pediatric patients 5 to 17 years of age with CKD on hemodialysis. The adverse reaction profile observed in pediatric patients was consistent with the safety profile found in adults. The safety and effectiveness of Mircera have not been established in patients less than 5 years of age [see Adverse Reactions (6.1) and Clinical Studies (14.2)].
The safety and effectiveness of Mircera have not been established in pediatric patients of any age for subcutaneous administration; for treatment of anemia in patients with CKD on peritoneal dialysis; for treatment of anemia in patients with CKD who are not yet on dialysis; and for patients whose hemoglobin level has not been previously stabilized by treatment with an ESA.
8.5 Geriatric Use
Clinical studies of Mircera did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function and of concomitant disease or other drug therapy.
-
10 OVERDOSAGE
Mircera overdosage can elevate hemoglobin levels above the desired level, which should be managed with discontinuation or reduction of Mircera dosage and/or with phlebotomy, as clinically indicated [see Pharmacodynamics (12.2)]. Cases of severe hypertension have been observed following overdose with ESAs [see Warnings and Precautions (5.3)].
-
11 DESCRIPTION
Methoxy polyethylene glycol-epoetin beta, is an ESA which differs from erythropoietin through formation of a chemical bond between either the N-terminal amino group or the ε-amino group of any lysine present in erythropoietin, predominantly Lys52 and Lys45, and methoxy polyethylene glycol (PEG) butanoic acid (approximately 30,000 daltons). This results in a total molecular weight of approximately 60,000 daltons. Methoxy polyethylene glycol-epoetin beta is produced in Chinese hamster ovary (CHO) cells using recombinant DNA technology.
Mircera is formulated as a sterile, preservative-free protein solution for intravenous or subcutaneous administration. Mircera (methoxy polyethylene glycol-epoetin beta) injection is supplied in prefilled syringes (30 mcg, 50 mcg, 75 mcg, 100 mcg, 120 mcg, 150 mcg, 200 mcg, or 250 mcg in 0.3 mL) and formulated in an aqueous solution containing mannitol (9 mg), methionine (0.447 mg), poloxamer 188 (0.03 mg), sodium phosphate monobasic monohydrate (0.414 mg), and sodium sulfate (1.704 mg). Mircera 360 mcg in 0.6 mL is formulated in an aqueous solution containing mannitol (18 mg), methionine (0.894 mg), poloxamer 188 (0.06 mg), sodium phosphate monobasic monohydrate (0.828 mg), and sodium sulfate (3.408 mg). The solution is clear, colorless to slightly yellowish and the pH is 6.2 ± 0.2.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Mircera is an erythropoietin receptor activator with greater activity in vivo as well as increased half-life, in contrast to erythropoietin. A primary growth factor for erythroid development, erythropoietin is produced in the kidney and released into the bloodstream in response to hypoxia. In responding to hypoxia, erythropoietin interacts with erythroid progenitor cells to increase red cell production. Production of endogenous erythropoietin is impaired in patients with CKD and erythropoietin deficiency is the primary cause of their anemia.
12.2 Pharmacodynamics
Following a single-dose of Mircera in adult patients with CKD, the onset of hemoglobin increase (defined as an increase more than 0.4 g/dL from baseline) was observed 7 to 15 days following initial dose administration [see Dosage and Administration (2.2)].
12.3 Pharmacokinetics
The pharmacokinetics of methoxy polyethylene glycol-epoetin beta were studied in adult anemic patients with CKD including patients on dialysis and those not on dialysis. Parameters are presented as the geometric mean [% coefficient of variation (%CV)] unless otherwise specified.
Methoxy polyethylene glycol-epoetin beta did not accumulate following administration every four weeks. However, when Mircera was administered every 2 weeks, serum concentrations at steady state increased by 12%. Multiple dosing had no effect on clearance, volume of distribution or bioavailability of methoxy polyethylene glycol-epoetin beta.
Absorption
Following the subcutaneous administration of Mircera, the maximum serum concentrations of methoxy polyethylene glycol-epoetin beta were observed at a median [min, max] time of 72 [24, 192] hours. The absolute bioavailability of Mircera after the subcutaneous administration was 62%.
Distribution
The volume of distribution at steady state was 61 [46%] mL/kg.
Elimination
Following an intravenous administration of Mircera 0.4 mcg/kg body weight to patients with CKD receiving peritoneal dialysis, the observed terminal half-life was 119 [55%] hours, and the total systemic clearance was 0.47 [35%] mL/hr/kg. Following a subcutaneous administration of Mircera 0.8 mcg/kg to CKD patients receiving peritoneal dialysis, the terminal half-life was 124 [57%] hours.
Specific Populations
The pharmacokinetics of methoxy polyethylene glycol-epoetin beta were not altered by age (range: 6 to 89 years), gender, race, severe hepatic impairment (Child-Pugh Class C), site of subcutaneous injection (abdomen, arm or thigh), or the use of dialysis.
Pediatric Patients
The pharmacokinetics of methoxy polyethylene glycol-epoetin beta were studied in 64 pediatric patients with CKD (ages 5 to 17 years) who were on hemodialysis and who were previously receiving another ESA (epoetin alfa/beta or darbepoetin alfa). At steady state (following the third intravenous administration of Mircera), the observed Cmax was 66 [150%] ng/mL, and the AUC0-tau was 7170 [140%] ng/hr/mL in the group who were dosed using the recommended conversion factor [see Dosage and Administration (2.2)]. Methoxy polyethylene glycol-epoetin beta serum concentrations declined with an apparent half-life of 121 [44%] hours, and a total systemic clearance of 0.51 [96%] mL/hr/kg.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
No carcinogenicity or genotoxicity studies have been conducted with Mircera. Methoxy polyethylene glycol-epoetin beta did not induce a proliferative response in either the erythropoietin receptor positive cell lines HepG2 and K562 or the erythropoietin receptor negative cell line RT112 in vitro. In addition, using a panel of human tissues, the in vitro binding of methoxy polyethylene glycol-epoetin beta was observed only in bone marrow progenitor cells.
When methoxy polyethylene glycol-epoetin beta was administered subcutaneously to male and female rats prior to and during mating, reproductive performance, fertility, and sperm assessment parameters were not affected.
-
14 CLINICAL STUDIES
14.1 Adult Patients
Patients with Chronic Kidney Disease on Dialysis: ESA Effects on Rates of Transfusion
In early clinical studies conducted in CKD patients on dialysis, ESAs have been shown to reduce the use of RBC transfusions. These studies enrolled patients with mean baseline hemoglobin levels of approximately 7.5 g/dL and ESAs were generally titrated to achieve a hemoglobin level of approximately 12 g/dL. Fewer transfusions were given during the ESA treatment period when compared to a pre-treatment interval.
In NHS, the yearly transfusion rate was 51.5% in the lower hemoglobin group (10 g/dL) and 32.4% in the higher hemoglobin group (14 g/dL).
Patients with Chronic Kidney Disease Not on Dialysis: ESA Effects on Rates of Transfusion
In TREAT, a randomized, double-blind trial of 4038 patients with CKD and type 2 diabetes not on dialysis, a post-hoc analysis showed that the proportion of patients receiving RBC transfusions was lower in patients administered an ESA to target a hemoglobin of 13 g/dL compared to the control arm in which the ESA was administered intermittently if hemoglobin concentration decreased to less than 9 g/dL (15% versus 25%, respectively). In CHOIR, a randomized open-label study of 1432 patients with CKD not on dialysis, use of an ESA to target a higher (13.5 g/dL) versus lower (11.3 g/dL) hemoglobin goal did not reduce the use of RBC transfusions. In each trial, no benefits occurred for the cardiovascular or end-stage renal disease outcomes. In each trial, the potential benefit of ESA therapy was offset by worse cardiovascular safety outcomes resulting in an unfavorable benefit-risk profile [see Warnings and Precautions (5.1)].
ESA Effects on Quality of Life
Mircera use has not been demonstrated in controlled clinical trials to improve quality of life, fatigue, or patient well-being.
ESA Effects on Rates of Death and Other Serious Cardiac Adverse Events
Three randomized outcome trials (NHS, CHOIR and TREAT) have been conducted in patients with CKD using Epogen/PROCRIT/Aranesp to target higher vs. lower hemoglobin levels. Though these trials were designed to establish a cardiovascular or renal benefit of targeting higher hemoglobin levels, in all 3 studies, patients randomized to the higher hemoglobin target experienced worse cardiovascular outcomes and showed no reduction in progression to ESRD. In each trial, the potential benefit of ESA therapy was offset by worse cardiovascular safety outcomes resulting in an unfavorable benefit-risk profile [see Warnings and Precautions (5.1)].
Other ESA Trials
The efficacy and safety of Mircera were assessed in six open-label, multi-center clinical studies that randomized patients to either Mircera or a comparator ESA. Two studies evaluated anemic patients with CKD who were not treated with an ESA at baseline and four studies evaluated patients who were receiving an ESA for treatment of the anemia of CKD. In all studies, patients were assessed as clinically stable at baseline and without evidence of infection or inflammation as determined by history and laboratory data, including C-reactive protein (CRP ≤ 15 mg/L for study 1 and CRP ≤ 30 mg/L for studies 2 to 6). A CRP value above the threshold led to the exclusion of no more than 3% of the screened patients.
In the clinical studies, ESAs were administered to achieve specific hemoglobin levels (see Table 6 and Table 7). Following stabilization of hemoglobin levels (12 g/dL), the median monthly Mircera dose was 150 mcg (range of 97 mcg to 270 mcg).
Patients Not Currently Treated with an ESA
In Study 1 patients who were not receiving dialysis were randomized to Mircera or darbepoetin alfa, administered for 28 weeks. The starting dose of Mircera was 0.6 mcg/kg administered subcutaneously once every two weeks and the starting dose of darbepoetin alfa was 0.45 mcg/kg administered subcutaneously once a week. In Study 2, patients who were receiving dialysis were randomized to Mircera or another ESA (epoetin alfa or epoetin beta), administered for 24 weeks. The starting dose of Mircera was 0.4 mcg/kg administered intravenously once every two weeks and the starting dose of the comparator was administered intravenously three times a week, consistent with the product’s recommended dose. In these studies, the observed median dose of Mircera once every two weeks over the course of the correction/evaluation period was 0.6 mcg/kg. Table 6 provides the results of the two studies.
Table 6 Clinical Studies in Patients Not Currently Treated with an ESA Group
(n)Percent Achieving Goal* (95% CI) Mean Hemoglobin Change from Baseline (g/dL) RBC Transfusion, % - * Goal: hemoglobin increase of at least 1 g/dL and to a level of at least 11 g/dL without RBC transfusion; hemoglobin levels were to be maintained within the range of 11 to 13 g/dL.
Study 1
Mircera
(n=162)98 (94, 99)
2.1
2.5
Darbepoetin alfa
(n=162)96 (92, 99)
2.0
6.8
Study 2
Mircera
(n=135)93 (88, 97)
2.7
5.2
Epoetin alfa/beta
(n=46)91 (79, 98)
2.6
4.3
Patients Currently Treated with an ESA
Four studies assessed the ability of Mircera to maintain hemoglobin levels among patients currently treated with other ESAs. Patients were randomized to receive Mircera administrations either once every two weeks or once every four weeks, or to continue their current ESA dose and schedule. The initial Mircera dose was determined based on the patient’s previous weekly ESA dose. As shown in Table 7, treatment with Mircera once every two weeks and once every four weeks maintained hemoglobin levels within the targeted hemoglobin range (10 to 13.5 g/dL).
Table 7 Clinical Studies in Patients Currently Treated with an ESA Group
(n)Mean Baseline Hemoglobin Evaluation Period Hemoglobin (Mean) Between-group Difference *,
g/dL (95% or 97.5% CI)*Mircera versus comparator mean hemoglobin difference in the evaluation period 97.5% CI are shown for
studies that compared two Mircera groups to another ESA (Studies 3 and 4) and 95% CI are shown for the
other studies.
n/a = not applicable
Data are from the per-protocol (primary analysis) population, numbers indicate total patients randomized.Study 3
Mircera intravenously every 2 weeks
(n=223)12.0
11.9
0.0 (−0.2, 0.2)
Mircera intravenously every 4 weeks
(n=224)11.9
11.9
0.1 (−0.2, 0.3)
Epoetin alfa/beta intravenously
(n=226)12.0
11.9
n/a
Study 4
Mircera subcutaneously every 2 weeks
(n=190)11.7
11.7
0.1 (−0.1, 0.4)
Mircera subcutaneously every 4 weeks
(n=191)11.6
11.5
−0.0 (−0.3, 0.2)
Epoetin beta subcutaneously
(n=191)11.6
11.5
n/a
Study 5
Mircera intravenously every 2 weeks
(n=157)12.0
12.1
0.2 (−0.0, 0.4)
Darbepoetin alfa intravenously
(n=156)11.9
11.8
n/a
Study 6
Mircera intravenously/ subcutaneously every 2 weeks
(n= 168)11.8
11.9
0.1 (−0.1, 0.4)
Epoetin alfa intravenously/ subcutaneously
(n=168)11.9
11.9
n/a
14.2 Pediatric Patients on Hemodialysis
Patients Currently Treated with an ESA
An open-label, multiple dose, multicenter, dose-finding study (NCT00717366) was conducted to determine the dose of intravenous Mircera when converting from treatment with another ESA (epoetin alfa/beta or darbepoetin alfa). The study was conducted in 64 pediatric patients (ages 5 to 17 years) with CKD who were on hemodialysis and who had stable hemoglobin (Hb) levels while previously receiving another ESA. Eligible patients were administered Mircera intravenously once every 4 weeks for 20 weeks. After the first administration of Mircera, dosage adjustments were permitted to maintain target Hb levels.
Efficacy was established based on the change in Hb concentration (g/dL) between the baseline and evaluation periods. Of the two conversion factors studied, the recommended conversion factor for Mircera [see Dosage and Administration (2.2)] was confirmed based on patients maintaining Hb within target levels. Among the 48 patients who received Mircera dosed using the recommended conversion factor, 9 patients withdrew due to renal transplant, 2 patients withdrew due to administrative reasons, 1 died, and 1 patient refused treatment. One of the 13 patients who withdrew from the study entered the evaluation period (weeks 17 to 21). For the 36 patients who entered the evaluation period and received the dose of Mircera calculated using the recommended conversion factor, the mean change in Hb concentration from baseline between the baseline and the evaluation period was -0.15 g/dL with 95% CI (-0.49, 0.2).
Supportive efficacy results in the group treated with Mircera using the recommended conversion factor demonstrated that 75% of patients maintained Hb values within ± 1 g/dL of baseline and 81% maintained Hb values within 10 to 12 g/dL during the evaluation period. Dose decreases and increases were reported in 38% of patients.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
Mircera (methoxy polyethylene glycol-epoetin beta) injection is available in single-dose prefilled syringes. The syringe plungers are designated with unique colors for each dosage strength. The prefilled syringes are supplied with a 27 gauge, ½ inch needle. To reduce the risk of accidental needlesticks after application, each prefilled syringe is equipped with a needle guard that covers the needle during disposal.
Mircera is available in the following pack sizes:
Single-Dose Prefilled Syringe with a Needle Guard.
A 27 Gauge, ½ inch Needle is also provided:
One Prefilled Syringe/Pack
NDC number
30 mcg/0.3 mL
NDC: 59353-400-09
50 mcg/0.3 mL
NDC: 59353-401-09
75 mcg/0.3 mL
NDC: 59353-402-09
100 mcg/0.3 mL
NDC: 59353-403-09
120 mcg/0.3 mL
NDC: 59353-407-09
150 mcg/0.3 mL
NDC: 59353-404-09
200 mcg/0.3 mL
NDC: 59353-405-09
250 mcg/0.3 mL
NDC: 59353-406-09
360 mcg/0.6 mL
NDC: 59353-408-09
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide and Instructions for Use).
Inform patients:
- Of the increased risks of mortality, serious cardiovascular reactions, thromboembolic reactions, stroke, and tumor progression [see Warnings and Precautions (5.1, 5.2)].
- To undergo regular blood pressure monitoring, adhere to prescribed anti-hypertensive regimen and follow recommended dietary restrictions.
- To seek medical care immediately if they experience any symptoms of an allergic reaction with use of Mircera [see Warnings and Precautions (5.7)].
- To contact their healthcare provider for new-onset neurologic symptoms or change in seizure frequency.
- Of the need to have regular laboratory tests for hemoglobin.
Administer Mircera under the direct supervision of a healthcare provider or, in situations where a patient has been trained to administer Mircera at home, provide instruction on the proper use of Mircera, including instructions to:
- Carefully review the Medication Guide and the Instructions for Use.
-
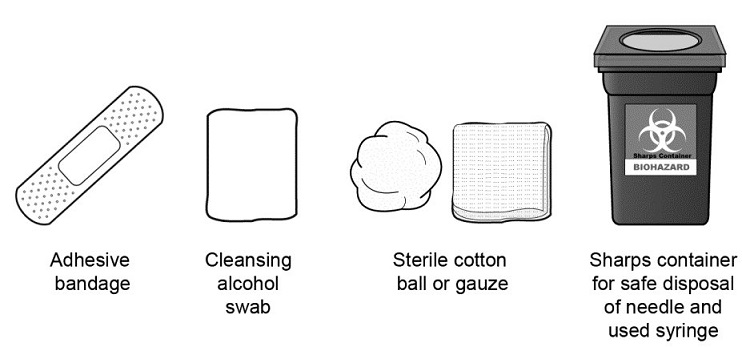
Avoid the reuse of needles, syringes, or unused portions of the Mircera single-dose prefilled syringes and to properly dispose of these items.
Always keep a puncture-proof disposal container available for the disposal of used syringes and needles.
Manufactured by:
Vifor (International) Inc.
Rechenstrasse 37
9014 St. Gallen
SwitzerlandU.S. License No. 2039
-
MEDICATION GUIDE
MIRCERA® (mir-SER-ah)
(methoxy polyethylene glycol-epoetin beta)Read this Medication Guide:
- before you start Mircera.
- if you are told by your healthcare provider that there is new information about Mircera.
- if you are told by your healthcare provider that you may inject Mircera at home, read this Medication Guide each time you receive a new supply of medicine.
This Medication Guide does not take the place of talking to your healthcare provider about your medical condition or your treatment. Talk with your healthcare provider regularly about the use of Mircera and ask if there is new information about Mircera.
What is the most important information I should know about Mircera?
Mircera may cause serious side effects that can lead to death, including:
For people with cancer:
- Mircera is not for use to treat anemia that is caused by cancer chemotherapy. If you have certain cancers, your tumor may grow faster and you may die sooner if you take Mircera.
For all people who take Mircera:
- Serious heart problems, such as heart attack or heart failure, and stroke. You may die sooner if you are treated with Mircera to increase red blood cells (RBCs) to near the same level found in healthy people.
-
Blood clots. Blood clots may happen at any time while taking Mircera. If you are receiving Mircera for any reason and you are going to have surgery, talk to your healthcare provider about whether or not you need to take a blood thinner to lessen the chance of blood clots during or following surgery. Blood clots can form in blood vessels (veins), especially in your leg (deep venous thrombosis or DVT). Pieces of a blood clot may travel to the lungs and block the blood circulation in the lungs (pulmonary embolus).
Call your healthcare provider or get medical help right away if you have any of these symptoms:- o Chest pain
- o Trouble breathing or shortness of breath
- o Pain in your legs, with or without swelling
- o A cool or pale arm or leg
- o Sudden confusion, trouble speaking, or trouble understanding others’ speech
- o Sudden numbness or weakness in your face, arm or leg, especially on one side of your body
- o Sudden trouble seeing
- o Sudden trouble walking, dizziness, loss of balance or coordination
- o Loss of consciousness (fainting)
- o Hemodialysis vascular access stops working
See “What are the possible side effects of Mircera?” below for more information.
If you decide to take Mircera, your healthcare provider should prescribe the smallest dose of Mircera that is necessary to reduce your chance of needing RBC transfusions.
What is Mircera?
Mircera is a prescription medicine used to treat anemia. People with anemia have a lower-than-normal number of RBCs. Mircera works like the human protein called erythropoietin to help your body make more RBCs. Mircera is used to reduce or avoid the need for RBC transfusions.
Mircera may be used to treat anemia if it is caused by chronic kidney disease (CKD) in:
- adults who may or may not be on hemodialysis, and
- children ages 5 to 17 years on hemodialysis.
If your hemoglobin level stays too high or if your hemoglobin goes up too quickly, this may lead to serious health problems which may result in death. These serious health problems may happen if you take Mircera, even if you do not have an increase in your hemoglobin level.
Mircera is not used for the treatment of anemia:
- that is caused by cancer chemotherapy
- in place of emergency treatment for anemia (RBC transfusions).
Mircera has not been proven to improve the quality of life, fatigue, or well-being.
It is not known if Mircera is safe and effective in children:
- for subcutaneous use
- below the age of 5 years
- receiving peritoneal dialysis or who are not yet treated with dialysis
- not already on an erythropoiesis-stimulating agent (ESA) therapy.
Do not take Mircera if you:
- Have high blood pressure that is not controlled (uncontrolled hypertension).
- Have been told by your healthcare provider that you have or have ever had a type of anemia called Pure Red Cell Aplasia (PRCA) that starts after treatment with Mircera or other erythropoietin protein medicines.
- Have had serious allergic reactions to Mircera.
Before taking Mircera, tell your healthcare provider about all of your medical conditions, including if you:
- Have heart disease.
- Have or develop cancer.
- Have high blood pressure.
- Have had a seizure (convulsion) or stroke.
- Receive dialysis treatment.
- Are pregnant or plan to become pregnant. It is not known if Mircera may harm your unborn baby.
- Are breastfeeding or plan to breastfeed. It is not known if Mircera passes into your breast milk.
Tell your healthcare provider about all of the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
How should I take Mircera?
-
If you or your caregiver has been trained to give Mircera shots (injections) at home:
- o Be sure that you read, understand, and follow the “Instructions for Use” that come with Mircera.
- o Take Mircera exactly as your healthcare provider tells you to. Do not change the dose of Mircera unless told to by your healthcare provider.
- o Your healthcare provider will show you how much Mircera to use, how to inject it, how often it should be injected, and how to safely throw away the used prefilled syringes and needles.
- o If you take more than your prescribed dose of Mircera, call your healthcare provider right away for instructions on what to do.
- During treatment with Mircera, continue to follow your healthcare provider’s instructions for diet, dialysis, and medicines, including medicines for high blood pressure.
- Have your blood pressure checked as instructed by your healthcare provider.
What are possible side effects of Mircera?
Mircera may cause serious side effects, including:
- See “What is the most important information I should know about Mircera?”
- High blood pressure. High blood pressure is a common side effect of Mircera in patients with chronic kidney disease. Your blood pressure may go up or be difficult to control with blood pressure medicine while taking Mircera. This can happen even if you have never had high blood pressure before. Your healthcare provider should check your blood pressure often. If your blood pressure does go up, your healthcare provider may prescribe new or more blood pressure medicine.
- Seizures. If you have any seizures while taking Mircera, get medical help right away and tell your healthcare provider.
- No response or loss of your hemoglobin response to Mircera. If your hemoglobin does not increase, or if the increase cannot be maintained, your healthcare provider will look for the cause of the problem. Your dose of Mircera or other medicines may need to be changed.
- Antibodies to Mircera. Your body may make antibodies to Mircera. These antibodies can block or lessen your body’s ability to make RBCs and cause you to have severe anemia. Call your healthcare provider if you have unusual tiredness, lack of energy, dizziness or fainting. You may need to stop taking Mircera.
- Serious allergic reaction. Serious allergic reactions can cause itching, shortness of breath, wheezing, dizziness and fainting because of a drop in blood pressure, swelling around your mouth or eyes, fast pulse, or sweating. If you have a serious allergic reaction, stop using Mircera and call your healthcare provider or get emergency medical help right away.
- Severe skin rash. Severe skin rash can occur. If you develop a new rash, call your healthcare provider right away.
Common side effects of Mircera include:
- diarrhea
- pain or swelling (inflammation) in your nose or throat (nasopharyngitis).
These are not all of the possible side effects of Mircera. Your healthcare provider can give you a more complete list. Tell your healthcare provider about any side effects that bother you or that do not go away.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store Mircera?
- Store Mircera prefilled syringes in the refrigerator at 36°F to 46°F (2°C to 8°C).
- Do not freeze or shake Mircera.
- Keep Mircera in the original carton to protect from light.
- Mircera prefilled syringes may be stored at room temperature up to 77°F (25°C) for no more than 30 days. Throw away (discard) Mircera after 30 days.
Keep Mircera and all medicines out of the reach of children.
General information about the safe and effective use of Mircera.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use Mircera for a condition for which it was not prescribed. Do not give Mircera to other people, even if they have the same symptoms that you have. It may harm them. You can ask your healthcare provider or pharmacist for information about Mircera that is written for health professionals.
What are the ingredients in Mircera?
Active ingredient: methoxy polyethylene glycol-epoetin beta
Inactive ingredients: mannitol, methionine, poloxamer 188, sodium phosphate monobasic monohydrate, and sodium sulfate.Manufactured by:
Vifor (International) Inc.
Rechenstrasse 37
9014 St. Gallen
SwitzerlandU.S. License No. 2039
U.S. Toll-free: 1-800–576-8295
Info_MirceraUS@viforpharma.com
Copyright © 2018 Vifor (International) Inc. All rights reserved.This Medication Guide has been approved by the U.S. Food and Drug Administration. Revised: 06/2018
-
Instructions for Use
MIRCERA® (mir-SER-ah)
(methoxy polyethylene glycol-epoetin beta)
Injection
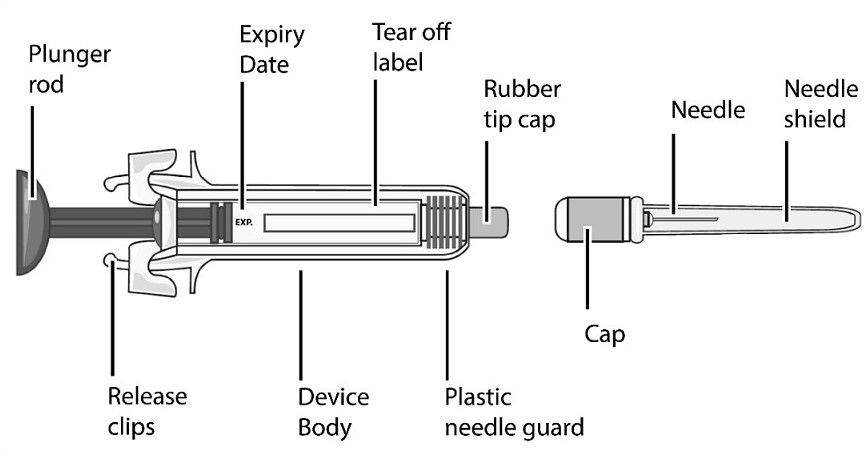
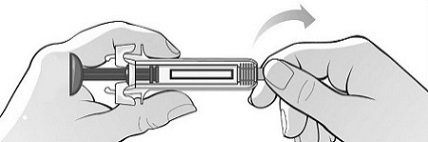
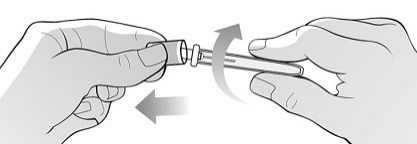
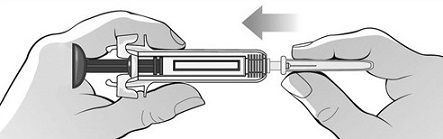
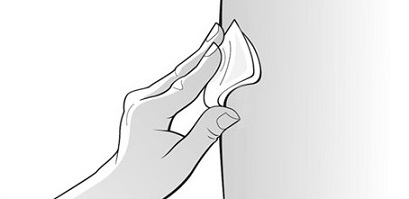
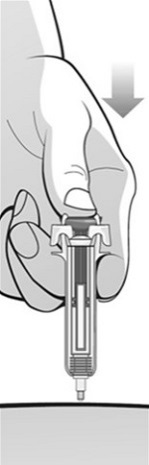
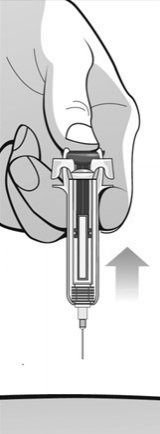
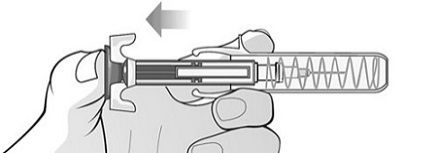
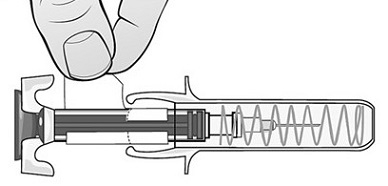
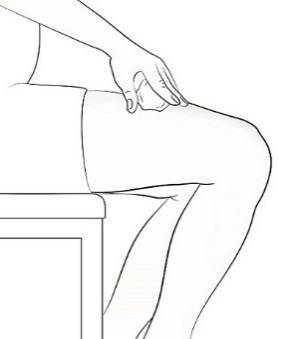
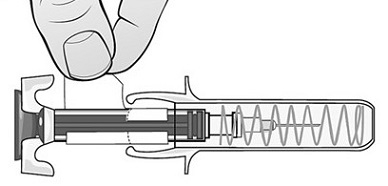
Single-Dose Prefilled SyringeRead the Medication Guide that comes with Mircera for the most important information you need to know. The following instructions explain how to give yourself or another individual an injection of Mircera using the single-dose prefilled syringe. Be sure that you read, understand and follow these instructions before injecting a dose of Mircera so that you are able to use the prefilled syringe correctly and safely. Your healthcare provider should show you how to correctly prepare and inject Mircera using the single-dose prefilled syringe before you use it for the first time. Ask your healthcare provider or pharmacist if you have any questions about Mircera.
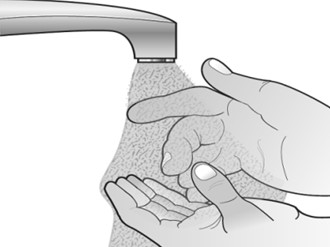
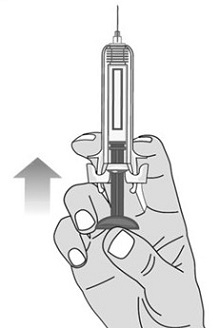
When you receive your Mircera prefilled syringes, make sure that:
- The name Mircera appears on the pack.
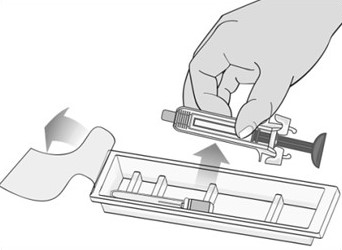
- The pack is not damaged. Do not use Mircera if the syringe, the pack or the plastic tray containing the syringe appears to be damaged.
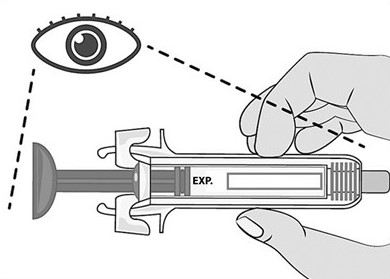
- The expiration date on the pack has not passed. Do not use Mircera after the expiration date on the pack.
-
The strength (mcg/mL) on the Mircera pack is the same as the strength (mcg/mL) prescribed by your healthcare provider. Mircera prefilled syringes come in several different strengths measured in mcg/mL. Depending on your prescribed dose you may:
- o receive more than one strength of Mircera prefilled syringes
- o need to give more than one injection to receive a complete dose
Important Information:
- Your healthcare provider may decide that you or a caregiver may give Mircera at home by an injection under your skin (subcutaneous) or by an intravenous (IV) injection. Your healthcare provider or nurse will train you how to prepare and inject Mircera properly. It is important that you do not try to give Mircera unless you or your caregiver has received training from your healthcare provider.
- Keep the Mircera prefilled syringe in the sealed pack until you are ready to use it.
- Do not hold or pull the prefilled syringe by the plunger.
- Do not remove the needle shield until you are ready to give an injection.
- Do not inject through clothing.
- Do not touch the release clips before use (see Figure A). Touching them may cause the needle safety device to be released too early and make the prefilled syringe unusable.
- Throw away (dispose of) the used Mircera prefilled syringe right away after use. Do not reuse a Mircera prefilled syringe or needle. See “Disposing of used prefilled syringes and needles” at the end of this Instructions for Use.
Storage
How should I store Mircera?
- Store Mircera in the refrigerator at 36°F to 46°F (2°C to 8°C).
- If a refrigerator is not available, Mircera prefilled syringes can be stored at room temperature 77°F or less (25°C or less) for no more than 30 days.
- Do not freeze Mircera. Do not use Mircera that has been frozen.
- Keep Mircera in the original package.
- Protect Mircera from light.
- Do not shake Mircera.
Keep Mircera and all medicines out of the reach of children.
Materials
Materials included in the Mircera prefilled syringe pack (Figure A):
- A prefilled syringe containing Mircera
- A separate injection needle
Figure A
Materials not included in the Mircera prefilled syringe pack (Figure B):
Figure B
Disposing of used prefilled syringes and needles:
- Put your used needles and prefilled syringes in an FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) loose needles and prefilled syringes in your household trash.
-
If you do not have an FDA-cleared sharps disposal container, you may use a household container that is:
- o made of heavy-duty plastic,
- o can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- o upright and stable during use,
- o leak-resistant, and
- o properly labeled to warn of hazardous waste inside the container.
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles, syringes and prefilled injectors. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA’s website at: http://www.fda.gov/safesharpsdisposal.
- Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
Always keep the sharps disposal container out of the reach of children.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Vifor (International) Inc., Rechenstrasse 37, 9014 St. Gallen, Switzerland, U.S. License No. 2039
U.S. Toll-free: 1-800–576-8295, Info_MirceraUS@viforpharma.com, Copyright © 2016 Vifor (International) Inc. All rights reserved.
Revised: April 2016
-
PRINCIPAL DISPLAY PANEL - 30 mcg / 0.3 mL Carton Label
NDC: 59353-400-09
MIRCERA®
(methoxy polyethylene glycol-epoetin beta)30 mcg / 0.3 mL
Sterile Solution
For intravenous or subcutaneous use only
Single-dose only. Discard unused portion.
Single-dose Prefilled Syringe with 27 Gauge NeedleKEEP REFRIGERATED
PHARMACIST: Each patient is required to receive the enclosed Medication Guide
Rx only
Vifor Pharma
-
PRINCIPAL DISPLAY PANEL - 50 mcg / 0.3 mL Carton Label
NDC: 59353-401-09
MIRCERA®
(methoxy polyethylene glycol-epoetin beta)50 mcg / 0.3 mL
Sterile Solution
For intravenous or subcutaneous use only
Single-dose only. Discard unused portion.
Single-dose Prefilled Syringe with 27 Gauge NeedleKEEP REFRIGERATED
PHARMACIST: Each patient is required to receive the enclosed Medication Guide
Rx only
Vifor Pharma
-
PRINCIPAL DISPLAY PANEL - 75 mcg / 0.3 mL Carton Label
NDC: 59353-402-09
MIRCERA®
(methoxy polyethylene glycol-epoetin beta)75 mcg / 0.3 mL
Sterile Solution
For intravenous or subcutaneous use only
Single-dose only. Discard unused portion.
Single-dose Prefilled Syringe with 27 Gauge NeedleKEEP REFRIGERATED
PHARMACIST: Each patient is required to receive the enclosed Medication Guide
Rx only
Vifor Pharma
-
PRINCIPAL DISPLAY PANEL - 100 mcg / 0.3 mL Carton Label
NDC: 59353-403-09
MIRCERA®
(methoxy polyethylene glycol-epoetin beta)100 mcg / 0.3 mL
Sterile Solution
For intravenous or subcutaneous use only
Single-dose only. Discard unused portion.
Single-dose Prefilled Syringe with 27 Gauge NeedleKEEP REFRIGERATED
PHARMACIST: Each patient is required to receive the enclosed Medication Guide
Rx only
Vifor Pharma
-
PRINCIPAL DISPLAY PANEL - 120 mcg / 0.3 mL Carton Label
NDC: 59353-407-09
MIRCERA®
(methoxy polyethylene glycol-epoetin beta)120 mcg / 0.3 mL
Sterile Solution
For intravenous or subcutaneous use only
Single-dose only. Discard unused portion.
Single-dose Prefilled Syringe with 27 Gauge NeedleKEEP REFRIGERATED
PHARMACIST: Each patient is required to receive the enclosed Medication Guide
Rx only
Vifor Pharma
-
PRINCIPAL DISPLAY PANEL - 150 mcg / 0.3 mL Carton Label
NDC: 59353-404-09
MIRCERA®
(methoxy polyethylene glycol-epoetin beta)150 mcg / 0.3 mL
Sterile Solution
For intravenous or subcutaneous use only
Single-dose only. Discard unused portion.
Single-dose Prefilled Syringe with 27 Gauge NeedleKEEP REFRIGERATED
PHARMACIST: Each patient is required to receive the enclosed Medication Guide
Rx only
Vifor Pharma
-
PRINCIPAL DISPLAY PANEL - 200 mcg / 0.3 mL Carton Label
NDC: 59353-405-09
MIRCERA®
(methoxy polyethylene glycol-epoetin beta)200 mcg / 0.3 mL
Sterile Solution
For intravenous or subcutaneous use only
Single-dose only. Discard unused portion.
Single-dose Prefilled Syringe with 27 Gauge NeedleKEEP REFRIGERATED
PHARMACIST: Each patient is required to receive the enclosed Medication Guide
Rx only
Vifor Pharma
-
PRINCIPAL DISPLAY PANEL - 250 mcg / 0.3 mL Carton Label
NDC: 59353-406-09
MIRCERA®
(methoxy polyethylene glycol-epoetin beta)250 mcg / 0.3 mL
Sterile Solution
For intravenous or subcutaneous use only
Single-dose only. Discard unused portion.
Single-dose Prefilled Syringe with 27 Gauge NeedleKEEP REFRIGERATED
PHARMACIST: Each patient is required to receive the enclosed Medication Guide
Rx only
Vifor Pharma
-
PRINCIPAL DISPLAY PANEL - 360 mcg / 0.6 mL Carton Label
NDC: 59353-408-09
MIRCERA®
(methoxy polyethylene glycol-epoetin beta)360 mcg / 0.6 mL
Sterile Solution
For intravenous or subcutaneous use only
Single-dose only. Discard unused portion.
Single-dose Prefilled Syringe with 27 Gauge NeedleKEEP REFRIGERATED
PHARMACIST: Each patient is required to receive the enclosed Medication Guide
Rx only
Vifor Pharma
-
INGREDIENTS AND APPEARANCE
MIRCERA
methoxy polyethylene glycol-epoetin beta injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 59353-400 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Methoxy polyethylene glycol-epoetin beta (UNII: LR3UXN0193) (Methoxy polyethylene glycol-epoetin beta - UNII:LR3UXN0193) Methoxy polyethylene glycol-epoetin beta 30 ug in 0.3 mL Inactive Ingredients Ingredient Name Strength SODIUM PHOSPHATE, MONOBASIC, UNSPECIFIED FORM (UNII: 3980JIH2SW) sodium sulfate anhydrous (UNII: 36KCS0R750) mannitol (UNII: 3OWL53L36A) methionine (UNII: AE28F7PNPL) poloxamer 188 (UNII: LQA7B6G8JG) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 59353-400-09 1 in 1 CARTON 10/24/2014 1 1 mL in 1 SYRINGE, GLASS; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125164 10/24/2014 MIRCERA
methoxy polyethylene glycol-epoetin beta injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 59353-401 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Methoxy polyethylene glycol-epoetin beta (UNII: LR3UXN0193) (Methoxy polyethylene glycol-epoetin beta - UNII:LR3UXN0193) Methoxy polyethylene glycol-epoetin beta 50 ug in 0.3 mL Inactive Ingredients Ingredient Name Strength SODIUM PHOSPHATE, MONOBASIC, UNSPECIFIED FORM (UNII: 3980JIH2SW) sodium sulfate anhydrous (UNII: 36KCS0R750) mannitol (UNII: 3OWL53L36A) methionine (UNII: AE28F7PNPL) poloxamer 188 (UNII: LQA7B6G8JG) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 59353-401-09 1 in 1 CARTON 10/24/2014 1 1 mL in 1 SYRINGE, GLASS; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125164 10/24/2014 MIRCERA
methoxy polyethylene glycol-epoetin beta injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 59353-402 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Methoxy polyethylene glycol-epoetin beta (UNII: LR3UXN0193) (Methoxy polyethylene glycol-epoetin beta - UNII:LR3UXN0193) Methoxy polyethylene glycol-epoetin beta 75 ug in 0.3 mL Inactive Ingredients Ingredient Name Strength SODIUM PHOSPHATE, MONOBASIC, UNSPECIFIED FORM (UNII: 3980JIH2SW) sodium sulfate anhydrous (UNII: 36KCS0R750) mannitol (UNII: 3OWL53L36A) methionine (UNII: AE28F7PNPL) poloxamer 188 (UNII: LQA7B6G8JG) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 59353-402-09 1 in 1 CARTON 10/24/2014 1 1 mL in 1 SYRINGE, GLASS; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125164 10/24/2014 MIRCERA
methoxy polyethylene glycol-epoetin beta injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 59353-403 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Methoxy polyethylene glycol-epoetin beta (UNII: LR3UXN0193) (Methoxy polyethylene glycol-epoetin beta - UNII:LR3UXN0193) Methoxy polyethylene glycol-epoetin beta 100 ug in 0.3 mL Inactive Ingredients Ingredient Name Strength SODIUM PHOSPHATE, MONOBASIC, UNSPECIFIED FORM (UNII: 3980JIH2SW) sodium sulfate anhydrous (UNII: 36KCS0R750) mannitol (UNII: 3OWL53L36A) methionine (UNII: AE28F7PNPL) poloxamer 188 (UNII: LQA7B6G8JG) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 59353-403-09 1 in 1 CARTON 10/24/2014 1 1 mL in 1 SYRINGE, GLASS; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125164 10/24/2014 MIRCERA
methoxy polyethylene glycol-epoetin beta injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 59353-407 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Methoxy polyethylene glycol-epoetin beta (UNII: LR3UXN0193) (Methoxy polyethylene glycol-epoetin beta - UNII:LR3UXN0193) Methoxy polyethylene glycol-epoetin beta 120 ug in 0.3 mL Inactive Ingredients Ingredient Name Strength SODIUM PHOSPHATE, MONOBASIC, UNSPECIFIED FORM (UNII: 3980JIH2SW) sodium sulfate anhydrous (UNII: 36KCS0R750) mannitol (UNII: 3OWL53L36A) methionine (UNII: AE28F7PNPL) poloxamer 188 (UNII: LQA7B6G8JG) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 59353-407-09 1 in 1 CARTON 10/24/2014 1 1 mL in 1 SYRINGE, GLASS; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125164 10/24/2014 MIRCERA
methoxy polyethylene glycol-epoetin beta injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 59353-404 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Methoxy polyethylene glycol-epoetin beta (UNII: LR3UXN0193) (Methoxy polyethylene glycol-epoetin beta - UNII:LR3UXN0193) Methoxy polyethylene glycol-epoetin beta 150 ug in 0.3 mL Inactive Ingredients Ingredient Name Strength SODIUM PHOSPHATE, MONOBASIC, UNSPECIFIED FORM (UNII: 3980JIH2SW) sodium sulfate anhydrous (UNII: 36KCS0R750) mannitol (UNII: 3OWL53L36A) methionine (UNII: AE28F7PNPL) poloxamer 188 (UNII: LQA7B6G8JG) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 59353-404-09 1 in 1 CARTON 10/24/2014 1 1 mL in 1 SYRINGE, GLASS; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125164 10/24/2014 MIRCERA
methoxy polyethylene glycol-epoetin beta injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 59353-405 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Methoxy polyethylene glycol-epoetin beta (UNII: LR3UXN0193) (Methoxy polyethylene glycol-epoetin beta - UNII:LR3UXN0193) Methoxy polyethylene glycol-epoetin beta 200 ug in 0.3 mL Inactive Ingredients Ingredient Name Strength SODIUM PHOSPHATE, MONOBASIC, UNSPECIFIED FORM (UNII: 3980JIH2SW) sodium sulfate anhydrous (UNII: 36KCS0R750) mannitol (UNII: 3OWL53L36A) methionine (UNII: AE28F7PNPL) poloxamer 188 (UNII: LQA7B6G8JG) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 59353-405-09 1 in 1 CARTON 10/24/2014 1 1 mL in 1 SYRINGE, GLASS; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125164 10/24/2014 MIRCERA
methoxy polyethylene glycol-epoetin beta injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 59353-406 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Methoxy polyethylene glycol-epoetin beta (UNII: LR3UXN0193) (Methoxy polyethylene glycol-epoetin beta - UNII:LR3UXN0193) Methoxy polyethylene glycol-epoetin beta 250 ug in 0.3 mL Inactive Ingredients Ingredient Name Strength SODIUM PHOSPHATE, MONOBASIC, UNSPECIFIED FORM (UNII: 3980JIH2SW) sodium sulfate anhydrous (UNII: 36KCS0R750) mannitol (UNII: 3OWL53L36A) methionine (UNII: AE28F7PNPL) poloxamer 188 (UNII: LQA7B6G8JG) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 59353-406-09 1 in 1 CARTON 10/24/2014 1 1 mL in 1 SYRINGE, GLASS; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125164 10/24/2014 MIRCERA
methoxy polyethylene glycol-epoetin beta injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 59353-408 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Methoxy polyethylene glycol-epoetin beta (UNII: LR3UXN0193) (Methoxy polyethylene glycol-epoetin beta - UNII:LR3UXN0193) Methoxy polyethylene glycol-epoetin beta 360 ug in 0.6 mL Inactive Ingredients Ingredient Name Strength SODIUM PHOSPHATE, MONOBASIC, UNSPECIFIED FORM (UNII: 3980JIH2SW) sodium sulfate anhydrous (UNII: 36KCS0R750) mannitol (UNII: 3OWL53L36A) methionine (UNII: AE28F7PNPL) poloxamer 188 (UNII: LQA7B6G8JG) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 59353-408-09 1 in 1 CARTON 10/24/2014 1 1 mL in 1 SYRINGE, GLASS; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125164 10/24/2014 Labeler - Vifor (International) Inc. (482603065) Establishment Name Address ID/FEI Business Operations Roche Diagnostics GmbH 323105205 API MANUFACTURE(59353-400, 59353-401, 59353-402, 59353-403, 59353-407, 59353-404, 59353-405, 59353-406, 59353-408) Establishment Name Address ID/FEI Business Operations Roche Diagnostics GmbH 315028860 MANUFACTURE(59353-400, 59353-401, 59353-402, 59353-403, 59353-407, 59353-404, 59353-405, 59353-406, 59353-408) , ANALYSIS(59353-400, 59353-401, 59353-402, 59353-403, 59353-407, 59353-404, 59353-405, 59353-406, 59353-408) Establishment Name Address ID/FEI Business Operations F. Hoffmann-La Roche Ltd. 485244961 MANUFACTURE(59353-400, 59353-401, 59353-402, 59353-403, 59353-407, 59353-404, 59353-405, 59353-406, 59353-408) , ANALYSIS(59353-400, 59353-401, 59353-402, 59353-403, 59353-407, 59353-404, 59353-405, 59353-406, 59353-408)
Trademark Results [Mircera]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 MIRCERA 88820445 not registered Live/Pending |
Genentech, Inc. 2020-03-04 |
 MIRCERA 87146117 not registered Live/Pending |
HOFFMANN-LA ROCHE INC. 2016-08-22 |
 MIRCERA 85888871 not registered Dead/Abandoned |
HOFFMANN-LA ROCHE INC. 2013-03-28 |
 MIRCERA 78304603 3237894 Dead/Cancelled |
Hoffmann-La Roche Inc. 2003-09-24 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.