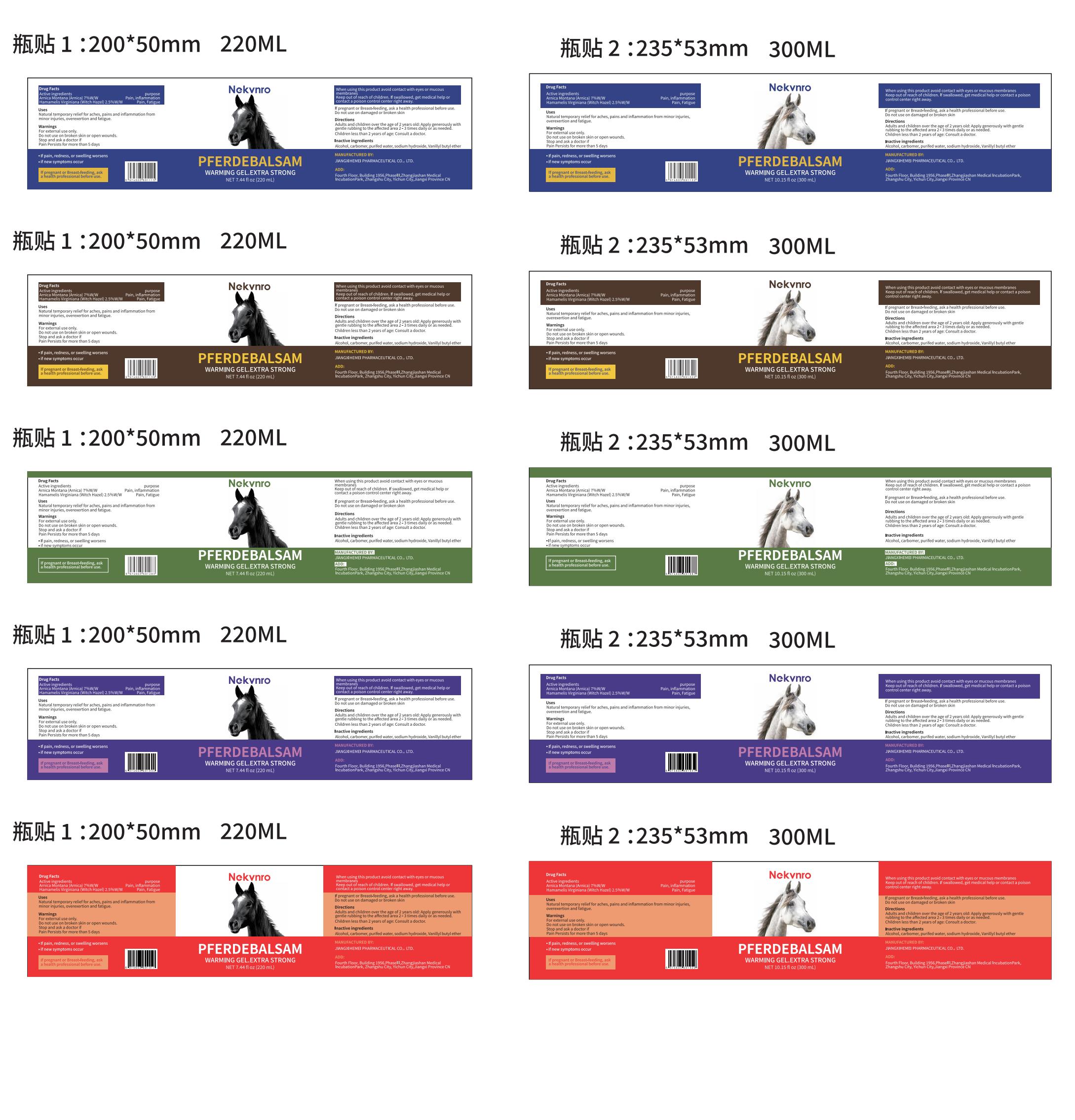
Nekvnro PFERDEBALSAM by Jiangxi Hemei Pharmaceutical Co., Ltd 84010-031 complete
Nekvnro PFERDEBALSAM by
Drug Labeling and Warnings
Nekvnro PFERDEBALSAM by is a Otc medication manufactured, distributed, or labeled by Jiangxi Hemei Pharmaceutical Co., Ltd. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
NEKVNRO PFERDEBALSAM- arnica montana(arnica) 7%, hamamelis virginiana (witch hazel) 2.5% pferdebalsam gel
Jiangxi Hemei Pharmaceutical Co., Ltd
----------
84010-031 complete
Use
Natural temporary relief for aches, pains and inflammation from minor injuries, overexertion and fatigue.
Warnings
For external use only.
Do not use on broken skin or open wounds.
Stop and ask a doctor if
Pain Persists for more than 5 days
.lf pain, redness, or swelling worsens
.if new symptoms occur
Ask Doctor
Pain Persists for more than 5 days
.lf pain, redness, or swelling worsens
.if new symptoms occur
Keep Oot Of Reach Of Children
lf swallowed, get medicalhelp or contact a poison control center right away.
| NEKVNRO PFERDEBALSAM
arnica montana(arnica) 7%, hamamelis virginiana (witch hazel) 2.5% pferdebalsam gel |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Jiangxi Hemei Pharmaceutical Co., Ltd (724892056) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Jiangxi Hemei Pharmaceutical Co., Ltd | 724892056 | manufacture(84010-031) | |