COLD SPOT POINT RELIEF- menthol swab
Cold Spot by
Drug Labeling and Warnings
Cold Spot by is a Otc medication manufactured, distributed, or labeled by Fabrication Enterprises, inc. , Pure Source . Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- ACTIVE INGREDIENT
-
INACTIVE INGREDIENT
aqua (deionized water), arnica montana flower (arnica) extract, chondroitin sulfate, citric acid, eucalyptus globulus oil, glucosamine sulfate,ilex paraguariensis leaf (yerba mate) extract, isopropyl alcohol, menth piperita (pepperment) oil, MSM (dimethyl sulfone) polysorbate-20, SD-alcohol 40B, triethanolamine
- KEEP OUT OF REACH OF CHILDREN
- WARNINGS
- INDICATIONS & USAGE
- DOSAGE & ADMINISTRATION
- PURPOSE
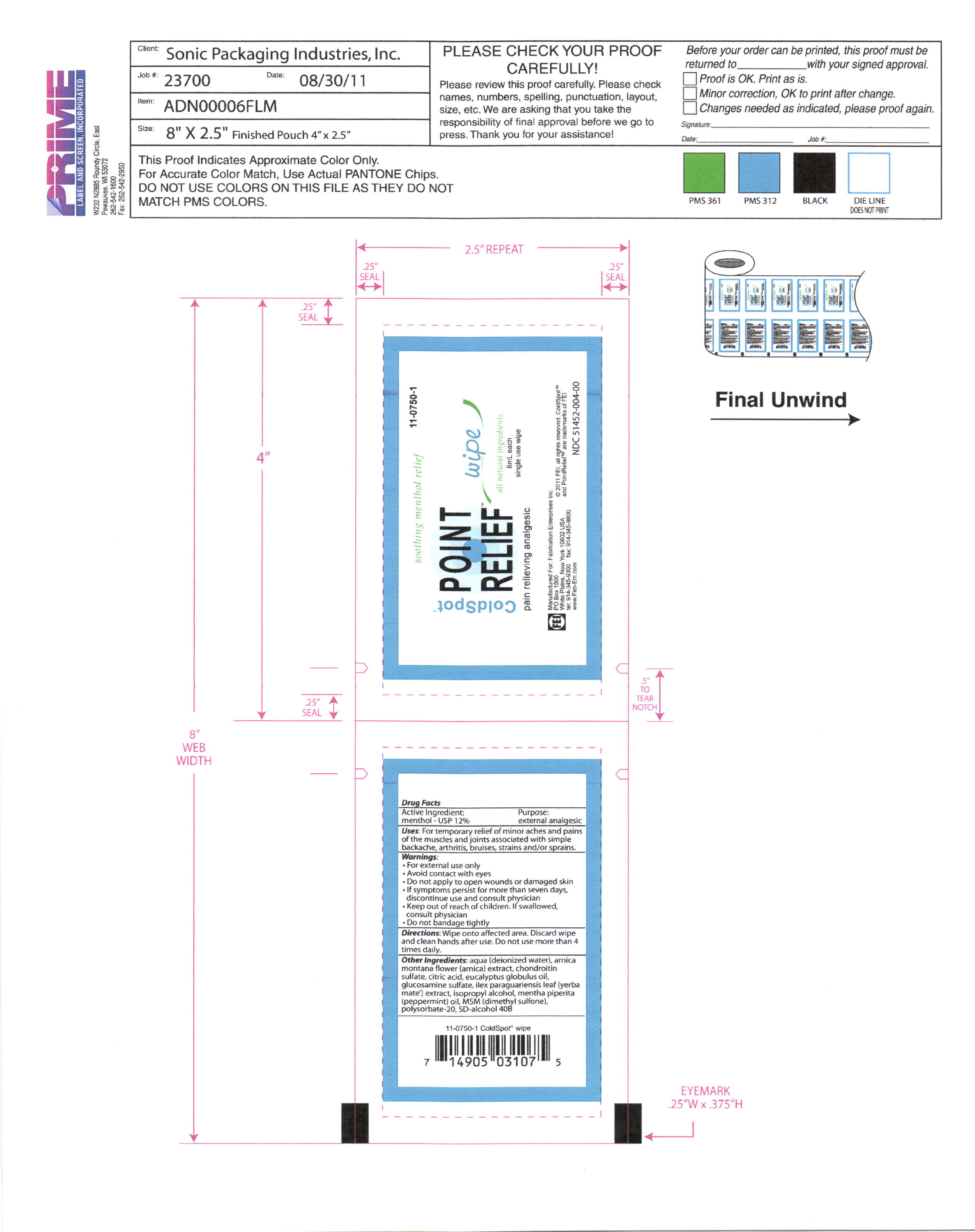
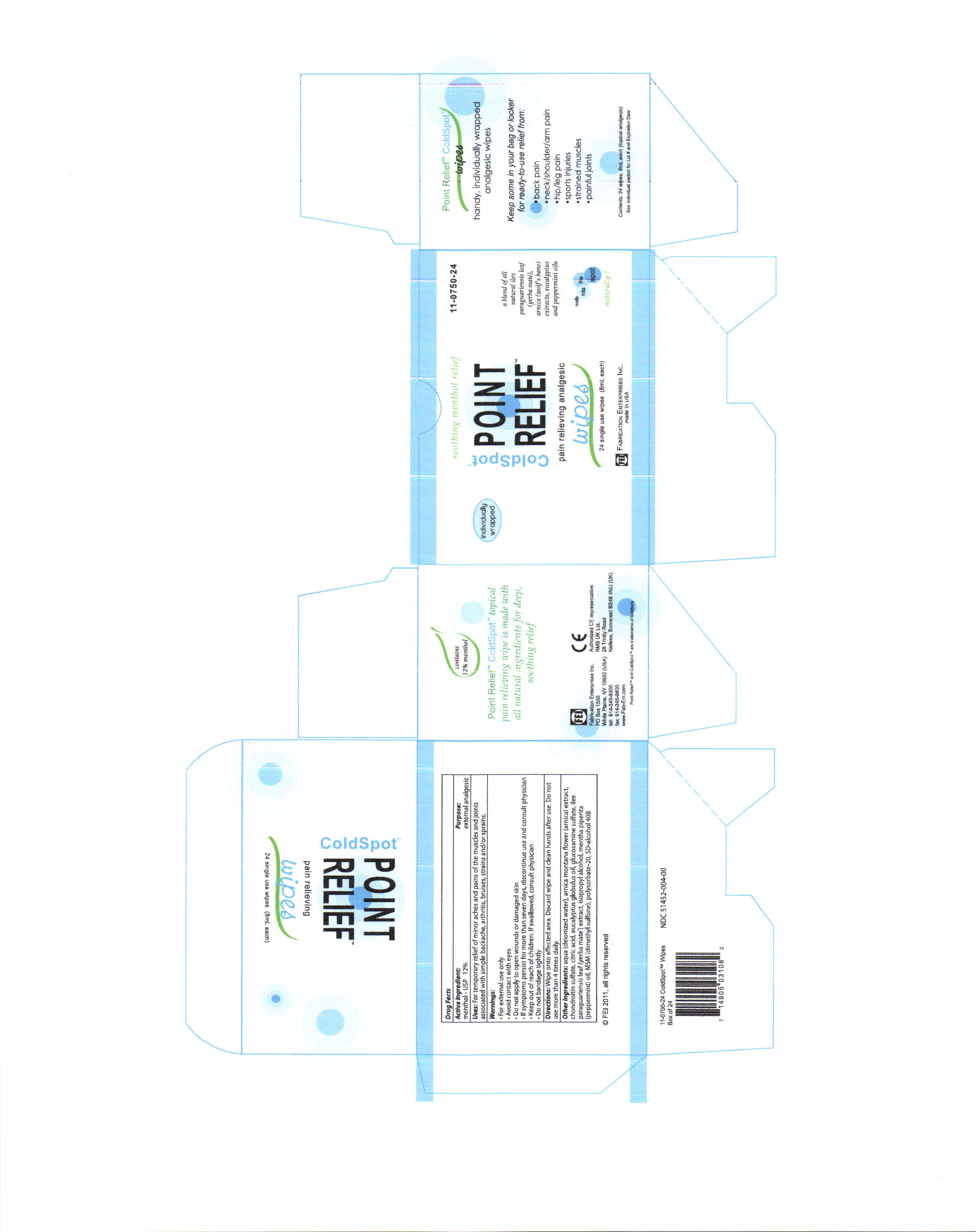
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
COLD SPOT POINT RELIEF
menthol swabProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 51452-004 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Menthol (UNII: L7T10EIP3A) (Menthol - UNII:L7T10EIP3A) Menthol .96 mL in 8 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ARNICA MONTANA FLOWER (UNII: OZ0E5Y15PZ) BOSWELLIA SERRATA RESIN OIL (UNII: 5T1XCE6K8K) BROMELAINS (UNII: U182GP2CF3) alcohol (UNII: 3K9958V90M) Eucalyptus Globulus leaf (UNII: S546YLW6E6) ILEX PARAGUARIENSIS LEAF (UNII: 1Q953B4O4F) Peppermint Oil (UNII: AV092KU4JH) Dimethyl Sulfone (UNII: 9H4PO4Z4FT) Chondroitin sulfate (bovine) (UNII: 6IC1M3OG5Z) Citric Acid (UNII: 2968PHW8QP) Isopropyl Alcohol (UNII: ND2M416302) polysorbate 20 (UNII: 7T1F30V5YH) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 51452-004-00 8 mL in 1 PACKET Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part341 10/13/2011 Labeler - Fabrication Enterprises, inc. (070577218) Registrant - Pure Source (969241041) Establishment Name Address ID/FEI Business Operations Pure Source 969241041 manufacture
Trademark Results [Cold Spot]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 COLD SPOT 78922431 not registered Dead/Abandoned |
Music Performance Laboratory, Inc. 2006-07-05 |
 COLD SPOT 78119312 2681344 Live/Registered |
Beekley Corporation 2002-04-03 |
 COLD SPOT 76040090 not registered Dead/Abandoned |
Connie Core-Dubay 2000-05-02 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.