Healcome Tattoo Numbing Cream 5% Lidocaine
Healcome Tattoo Numbing Cream 5% Lidocaine by
Drug Labeling and Warnings
Healcome Tattoo Numbing Cream 5% Lidocaine by is a Otc medication manufactured, distributed, or labeled by YITONGBADA (SHENZHEN) INTERNATIONAL TRADE CO., LTD. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
HEALCOME TATTOO NUMBING CREAM 5% LIDOCAINE- lidocaine cream
YITONGBADA (SHENZHEN) INTERNATIONAL TRADE CO., LTD
----------
Healcome Tattoo Numbing Cream 5% Lidocaine
Drug Facts
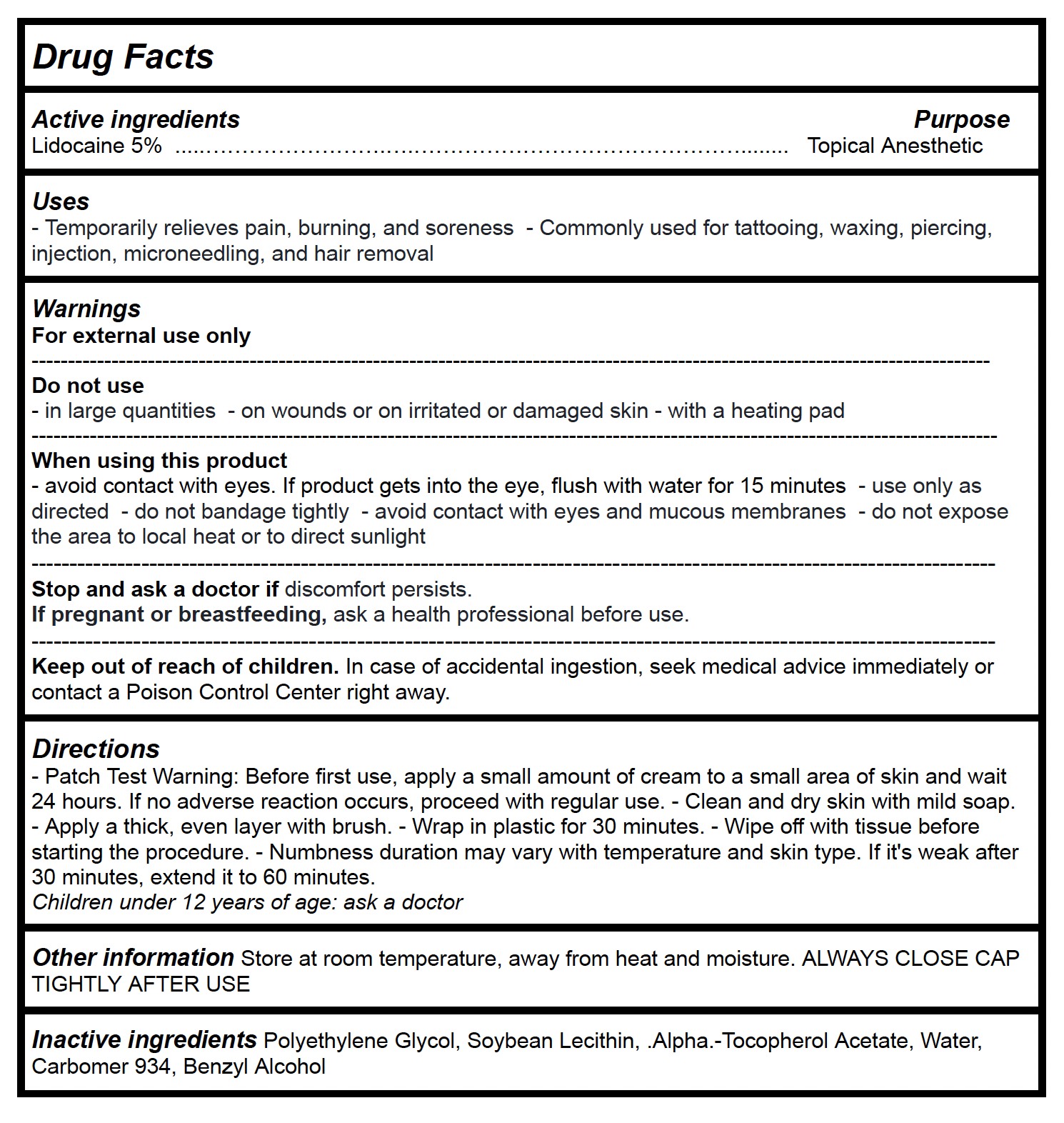
Uses
- Temporarily relieves pain, burning, and soreness - Commonly used for tattooing, waxing, piercing, injection, microneedling, and hair removal
Directions
- Patch Test Warning: Before first use, apply a small amount of cream to a small area of skin and wait 24 hours. If no adverse reaction occurs, proceed with regular use. - Clean and dry skin with mild soap. - Apply a thick, even layer with brush. - Wrap in plastic for 30 minutes. - Wipe off with tissue before starting the procedure. - Numbness duration may vary with temperature and skin type. If it's weak after 30 minutes, extend it to 60 minutes.
Children under 12 years of age: ask a doctor
| HEALCOME TATTOO NUMBING CREAM 5% LIDOCAINE
lidocaine cream |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - YITONGBADA (SHENZHEN) INTERNATIONAL TRADE CO., LTD (725220463) |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.