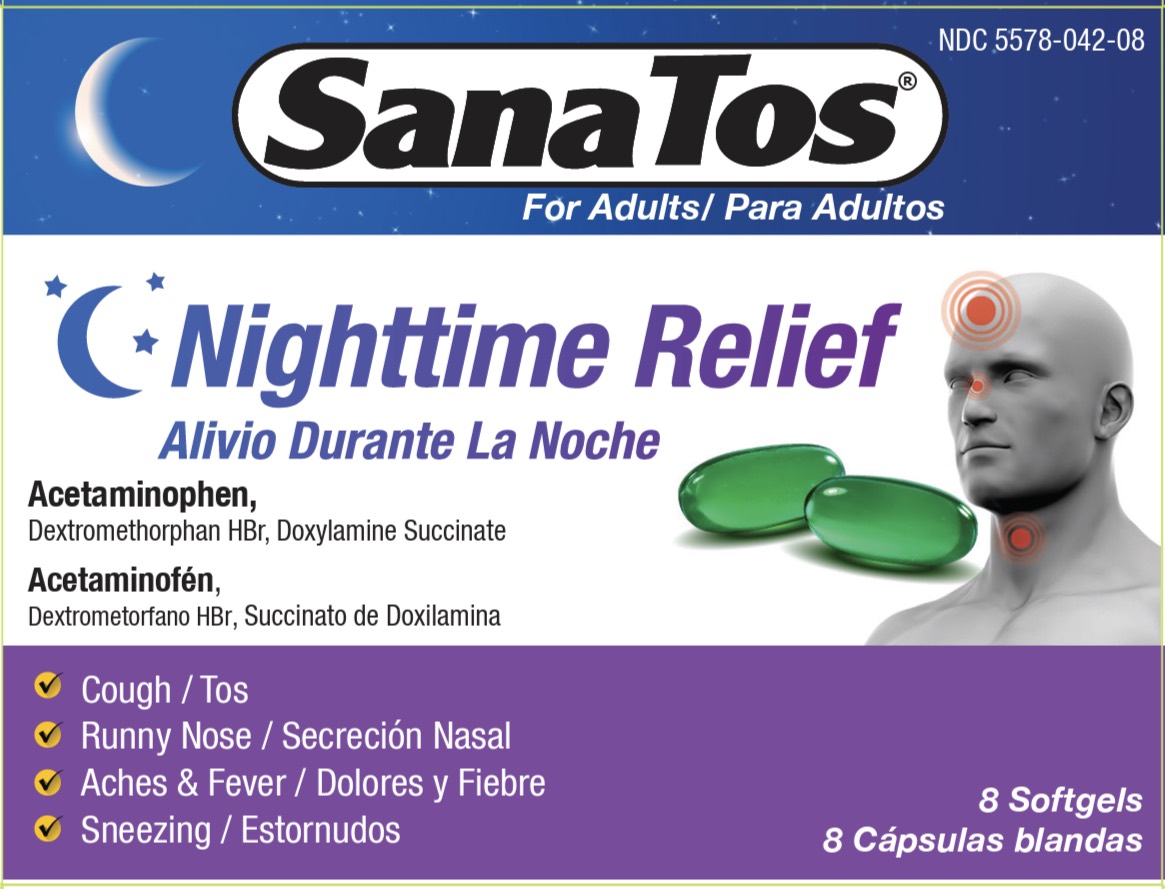
Sanatos Nightime Softgel (HP)
Sanatos by
Drug Labeling and Warnings
Sanatos by is a Otc medication manufactured, distributed, or labeled by Pharmadel LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
SANATOS NIGHTTIME- acetaminophen, dextromethorphan hbr, doxylamine succinate capsule, liquid filled
Pharmadel LLC
----------
Sanatos Nightime Softgel (HP)
Uses
Temporarily relieves common cold/flu symptoms
- cough due to minor throat and bronchial irritation
- headache
- minor aches and pains
- fever
- runny nose
- sneezing
- itching of the nose or throat
- itchy, watery eyes due to hay fever
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if you take
- more than 4 doses in 24 hours, which is the maximum daily amount for this product
- with other drugs containing acetaminophen
- 3 or more alcoholic drinks daily while using this product
Allergy alert: Acetaminophen may cause severe skin reactions. Symptoms may include:
- skin reddening
- blisters
- rash
If a skin reaction occurs, stop use and seek medical help right away.
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product. for more than 3 days for fever unless directed by a doctor
Ask a doctor before use if you have
- liver disease
- glaucoma
- a cough that is accompanied by excessive phlegm (mucus)
- a breathing problem, persistent or chronic cough as occurs with smoking, asthma, chronic bronchitis or emphysema
- trouble urinating due to enlarged prostate gland
Ask a doctor or pharmacist before use if you are
- taking sedatives or tranquilizers
- taking the blood thinning drug warfarin
When using this product
- may cause marked drowsiness
- excitability may occur, especially in children
- may cause marked drowsiness; alcohol, sedatives and tranquilizers may increase drowsiness effect
- avoid alcoholic drinks when taking this product
- use caution when driving a motor vehicle or operating machinery
Directions
- do not exceed recommended dosage (see Liver warning)
- do not exceed 4 doses in a 24 hour period or as directed by a doctor
| Age | Dose |
| adults and children 12 years and older | 2 softgels every 6 hours |
| children 4 to under 12 years | consult a doctor |
| children under 4 years | do not use |
Other information
- store between 68-77°F (20-25°C)
- avoid excessive heat
- do not use if blister pack is punctured or torn
| SANATOS
NIGHTTIME
acetaminophen, dextromethorphan hbr, doxylamine succinate capsule, liquid filled |
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
 |
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| Labeler - Pharmadel LLC (030129680) |
Revised: 8/2025
Document Id: 3d59b09c-5639-eebb-e063-6394a90aa7c6
Set id: 22f53268-8979-c34e-e063-6394a90a668d
Version: 4
Effective Time: 20250827
Trademark Results [Sanatos]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 SANATOS 77703500 3887159 Live/Registered |
PHARMADEL, LLC 2009-03-31 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.