ANTI-AGING SUNSCREEN BROAD SPECTRUM SPF 30
ANTI-AGING SUNSCREEN BROAD SPECTRUM SPF 30 by
Drug Labeling and Warnings
ANTI-AGING SUNSCREEN BROAD SPECTRUM SPF 30 by is a Otc medication manufactured, distributed, or labeled by La Prairie, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
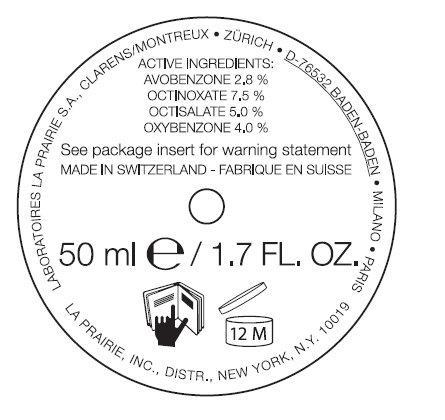
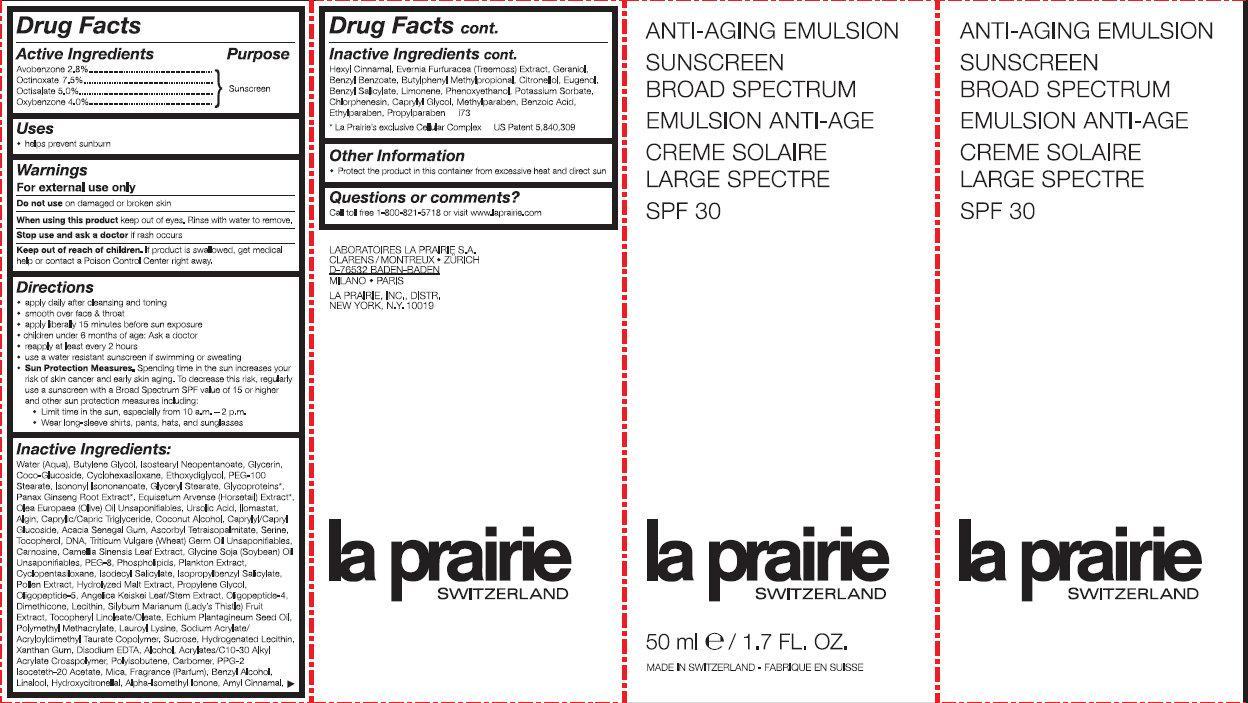
ANTI-AGING SUNSCREEN BROAD SPECTRUM SPF 30- avobenzone, octinoxate, octisalate, oxybenzone emulsion
La Prairie, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
ANTI-AGING SUNSCREEN BROAD SPECTRUM SPF 30
Keep out of reach of children.
If product is swallowed, get medical help or contact a Poison Control Center right away.
Directions:
apply daily after cleansing and toning smooth over face and throat apply liberally 15 minutes before sun exposure children under 6 months of age: Ask a doctor reapply at least every 2 hours use a water resistant sunscreen if swimming or sweating Sun Protection Measures. Spending time in the sun increase your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including: Limit time in the sun, especially from 10 a.m. - 2 p.m. Wear long-sleeve shirts, pants, hats and sunglasses
Inactive Ingredients:
Water(Aqua), Butylene Glycol, Isostearyl Neopentanoate, Glycerin, Coco-Glucoside, Cyclohexasiloxane, Ethoxydiglycol, Peg-100 Stearate, Isononyl Isononanoate, Glyceryl Stearate, Glycoproteins*, Panax Ginseng Root Extract*, Equisetum Arvense (Horsetail) Extract, Olea Europaea (Olive) Oil Unsapoinfiables, Ursolic Acid, Llomastat, Algin, Caprylic/Capric Triglyceride, Coconut Alcohol, Caprylic/Capryl Glucoside, Acacia Senegal Gum, Ascorbyl Tetraisopalmitate, Serine, Tocopherol, DNA, Triticum Vulgare (Wheat) Germ Oil Unsaponifiables, Carnosine, Camellia Sinensis Leaf Extract, Glycine Soja (Soybean) Oil Unsaponifiables, PEG-8, Phospholipids, Plankton Extract, Cyclopentasiloxane, Isodecyl Salicylate, Isopropylbenzyl Salicylate, Pollen Extract, Hydrolyzed Malt Extract, Propylene Glycol, Oligopeptide-5, Angelica Keiskei Extract, Oligopeptide-4, Dimethicone, Lecithin, Silybum Marianum (Lady's Thistle) Fruit Extract, Tocopheryl Linoleate/Oleate, Echium Plantagineum Seed Oil, Polymethyl Methacrylate, Lauroyl Lysine, Sodium Acrylate/Acryloyldimethyl Taurate Copolymer, Sucrose, Xanthan Gum, Disodium EDTA, Alcohol, Acrylates/C10-30 Alkyl Acrylate Crosscopolymer, Polyisobutene, Carbomer, PPG-2 Isoceteth-20 Acetate, Mica, Fragrance (Parfum), Benzyl Alcohol, Linalool, Hydroxycitronellal, Alpha-Isomethyl Ionone, Amyl Cinnamal, Hexyl Cinnamal, Evernia Furfuracea (Treemoss) Extract, Geraniol, Benzyl Benzoate, Butylphenyl Methylpropiona, Citronellol, Eugenol, Benzyl Salicylate, Limonene, Phenoxyethanol, Potassium Sorbate, Chlorphenesin, Methylparaben, Benzoic Acid, Ethylparaben *La Prairie’s exclusive Cellular Complex US Patent 5,840,309
| ANTI-AGING SUNSCREEN BROAD SPECTRUM SPF 30
avobenzone, octinoxate, octisalate, oxybenzone emulsion |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - La Prairie, Inc. (606554996) |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.