AZMIRO- testosterone cypionate injection, solution
AZMIRO by
Drug Labeling and Warnings
AZMIRO by is a Prescription medication manufactured, distributed, or labeled by Azurity Pharmaceuticals, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use AZMIRO safely and effectively. See full prescribing information for AZMIRO.
AZMIRO TM (testosterone cypionate) injection, for intramuscular use, CIII
Initial U.S. Approval: 1953RECENT MAJOR CHANGES
INDICATIONS AND USAGE
AZMIRO is an androgen indicated for testosterone replacement therapy in males for conditions associated with a deficiency or absence of endogenous testosterone ( 1):
Limitations of Use:
Safety and efficacy of AZMIRO in men with “age-related hypogonadism” (also referred to as “late-onset hypogonadism”) have not been established ( 1).
Safety and effectiveness in pediatric patients below the age of 12 years have not been established ( 8.4).DOSAGE AND ADMINISTRATION
Injectable testosterone products may have different doses, strengths, or administration instructions and they are not substitutable on a milligram-per-milligram basis. Administer AZMIRO by deep gluteal intramuscular injection only ( 2.1).
Prior to initiating AZMIRO, confirm the diagnosis of hypogonadism by ensuring that serum testosterone concentrations have been measured in the morning on at least two separate days and that these serum concentrations are below the normal range ( 2.2).
Recommended dosage is 50 mg to 400 mg administered every two to four weeks as a deep intramuscular injection in the gluteal muscle.
Individualize the dose and schedule based on the patient’s age, diagnosis, response to treatment, and the appearance of adverse reactions ( 2.3).The prefilled syringe should be administered as an intramuscular injection by a healthcare professional only.
DOSAGE FORMS AND STRENGTHS
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
Polycythemia: Monitor hematocrit periodically during treatment. Discontinue AZMIRO, if necessary ( 5.1).
Venous thromboembolism (VTE): VTE, including deep vein thrombosis (DVT) and pulmonary embolism (PE) have been reported in patients using testosterone products. Discontinue AZMIRO if VTE is suspected and initiate appropriate workup and management ( 5.2).
Worsening of Benign Prostatic Hyperplasia (BPH) and Potential Risk of Prostate Cancer: Monitor patients with benign prostatic hyperplasia (BPH) for worsening of signs and symptoms of BPH. Evaluate patients for prostate cancer, including monitoring prostate specific antigen (PSA) prior to initiating and during treatment with androgens ( 5.3).Blood Pressure Increases: Testosterone can increase blood pressure, which can increase cardiovascular risk over time. Measure blood pressure periodically. Not recommended for use in men with uncontrolled hypertension (5.4)
Abuse of Testosterone and Monitoring of Serum Testosterone: If testosterone use at doses higher than recommended for the approved indication and in combination with other anabolic androgenic steroids is suspected, check serum testosterone concentration ( 5.5)
Potential for Adverse Effects on Spermatogenesis: AZMIRO may cause azoospermia ( 5.7, 8.3).
Edema: Edema, with or without congestive heart failure (CHF), may occur in patients with pre-existing cardiac, renal, or hepatic disease. Discontinue AZMIRO and initiate appropriate workup ( 5.9).
Sleep Apnea: AZMIRO may potentiate sleep apnea in those with risk factors ( 5.10).
Lipid Changes: Testosterone may affect serum lipid profile. Monitor patient lipid concentrations; if necessary, adjust dosage of lipid lowering drug(s) or discontinue AZMIRO ( 5.12).
Adverse Effects on Bone Maturation: Testosterone may result in acceleration of bone age and premature closure of epiphyses in pediatric patients which may result in compromised adult stature. Monitor the effect on bone maturation by assessing bone age of the wrist and hand every 6 months.ADVERSE REACTIONS
Common adverse reactions (incidence ≥4%) are injection site erythema and injection site reaction ( 6.1).
Other adverse reactions include: polycythemia, gynecomastia, headache, and depression ( 6.2).
To report SUSPECTED ADVERSE REACTIONS, contact Azurity Pharmaceuticals, Inc. at 1-800-461-7449 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.DRUG INTERACTIONS
Insulin: In patients with diabetes, concomitant use with AZMIRO may decrease blood glucose and insulin requirements ( 7.1).
Oral Anticoagulants: Concomitant use with AZMIRO may cause changes in anticoagulant activity. Monitor International Normalized Ratio and prothrombin time frequently ( 7.2).
Corticosteroids: Concomitant use with AZMIRO may result in increased fluid retention. Use with caution, particularly in patients with cardiac, renal, or hepatic disease ( 7.3).USE IN SPECIFIC POPULATIONS
Geriatric Patients: Geriatric patients treated with androgens may also be at risk for worsening of signs and symptoms of BPH and prostatic carcinoma ( 8.5).
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 7/2025
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS & USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Important Dosage Information
2.2 Confirmation of Hypogonadism before Initiation of AZMIRO
2.3 Recommended Dosage
2.4 Administration Instructions for AZMIRO Single-dose Vial
2.5 Important Administration Information and Administration Instructions for AZMIRO Prefilled Syringe
3 DOSAGE FORMS & STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Polycythemia
5.2 Venous Thromboembolism (VTE)
5.3 Worsening of Benign Prostatic Hyperplasia (BPH) and Potential Risk of Prostate Cancer
5.4 Blood Pressure Increases
5.5 Abuse of Testosterone and Monitoring of Serum Testosterone Concentrations
5.6 Not for Use in Women
5.7 Potential for Adverse Effects on Spermatogenesis
5.8 Hepatic Adverse Effects
5.9 Edema
5.10 Sleep Apnea
5.11 Gynecomastia
5.12 Lipid Changes
5.13 Hypercalcemia
5.14 Decreased Thyroxine-binding Globulin
5.15 Increases in Prolactin
5.16 Adverse Effects on Bone Maturation
6 ADVERSE REACTIONS
6.1 Clinical Trial Experience
6.2 Other Adverse Reactions
7 DRUG INTERACTIONS
7.1 Insulin
7.2 Oral Anticoagulants
7.3 Corticosteroids
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
9 DRUG ABUSE AND DEPENDENCE
9.1 Controlled Substance
9.2 Abuse
9.3 Dependence
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS & USAGE
AZMIRO is indicated for testosterone replacement therapy in males in conditions associated with a deficiency or absence of endogenous testosterone:
Primary hypogonadism (congenital or acquired): testicular failure due to conditions such as cryptorchidism, bilateral torsion, orchitis, vanishing testis syndrome; or orchiectomy, Klinefelter’s syndrome, or toxic damage from alcohol or heavy metals, chemotherapy, or toxic damage from alcohol or heavy metals. These men usually have low serum testosterone concentrations and gonadotropins (follicle stimulating hormone (FSH), luteinizing hormone (LH)) above the normal range [ see Dosage and Administration ( 2.2) ].
Hypogonadotropic hypogonadism (congenital or acquired): gonadotropin or luteinizing hormone-releasing hormone (LHRH) deficiency, or pituitary-hypothalamic injury from tumors, trauma, or radiation. These men have low testosterone serum concentrations but have gonadotropins in the normal or low range [ see Dosage and Administration (2.2)].
Limitations of Use
Safety and efficacy of AZMIRO in men with “age- related hypogonadism” (also referred to as “late-onset hypogonadism”) have not been established.
Safety and efficacy of AZMIRO in pediatric patients below the age of 12 years have not been established [ see Use in Specific Populations ( 8.4) ]. -
2 DOSAGE AND ADMINISTRATION
2.1 Important Dosage Information
Injectable testosterone products may have different doses, strengths, or administration instructions and they are not substitutable on a milligram-per-milligram basis.
Administer AZMIRO by deep gluteal intramuscular injection only.2.2 Confirmation of Hypogonadism before Initiation of AZMIRO
Prior to initiating AZMIRO, confirm the diagnosis of hypogonadism by ensuring that serum testosterone concentrations have been measured in the morning on at least two separate days and that these serum testosterone concentrations are below the normal range.
2.3 Recommended Dosage
The recommended dosage of AZMIRO is 50 mg to 400 mg administered every two to four weeks as a deep intramuscular injection in the gluteal muscle.
Individualize the dose and schedule of AZMIRO based on the patient’s age, diagnosis, response to treatment, and the appearance of adverse reactions.2.4 Administration Instructions for AZMIRO Single-dose Vial
Visually inspect parenteral drug products for particulate matter and discoloration prior to administration, whenever solution and container permit.
Discard any unused portions of drug remaining in the single-dose vial.2.5 Important Administration Information and Administration Instructions for AZMIRO Prefilled Syringe
Important Administration Information
The prefilled syringe should be administered as an intramuscular injection by a healthcare professional only.
The prefilled syringe should only be used to administer doses of 200 mg. For administration of doses other than 200 mg, use the AZMIRO single-dose vial.
AZMIRO 200 mg/mL is supplied as single-dose prefilled syringe with luer lock connector.
The prefilled syringe carton does not contain a needle. Obtain suitable needle separately.
Do not use if the packaging appears to be damaged.
Do not use the prefilled syringe if it appears to be damaged or the syringe cap is detached from the Luer lock.
Administration Instructions
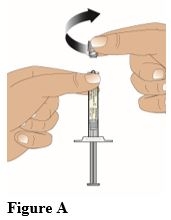
Hold the syringe upright while gripping the ribbed part of the luer lock. Then with the other hand unscrew the syringe cap in a counter-clockwise direction (Figure A). Once the syringe cap is removed, avoid any hand contact with the tip of the glass syringe, to maintain the sterility.
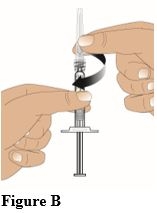
Screw the needle onto the luer lock end of the syringe in a clockwise direction until it is firmly attached (Figure B).
Remove the needle cap by pulling it straight off.
Check syringe for air bubbles. If there are air bubbles: Gently tap the syringe with your fingers until the bubbles rise to the top of the syringe. Then press the plunger up to slowly expel the air.
Clean the injection site for injection into the gluteal muscle with an alcohol swab and allow the skin to dry.
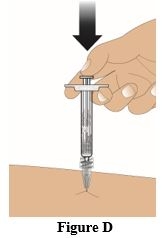
Insert the needle straight into the skin at a 90° angle (Figure C). Push the plunger down to inject the entire contents (1 mL) of the prefilled syringe (Figure D).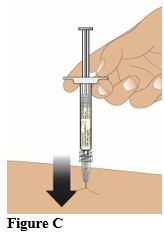
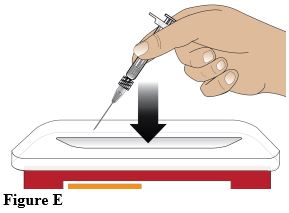
After administration of the injection, discard the syringe into a sharps disposal container or in accordance with local requirements (Figure E).
- 3 DOSAGE FORMS & STRENGTHS
-
4 CONTRAINDICATIONS
AZMIRO is contraindicated in:
Known hypersensitivity to AZMIRO or to any of its components [see Description ( 11)]. Hypersensitivity, including skin manifestations and anaphylactoid reactions have been reported [ see Adverse Reactions ( 6.2) ].
Men with carcinoma of the breast or known or suspected carcinoma of the prostate gland [ see Warnings and Precautions ( 5.3) ].
Women who are pregnant. Testosterone can cause virilization of the female fetus when administered to a pregnant woman [ see Use in Specific Populations ( 8.1) ]. -
5 WARNINGS AND PRECAUTIONS
5.1 Polycythemia
Increases in hematocrit levels, reflective of increases in red blood cell mass, may require discontinuation of AZMIRO. Check that hematocrit is not elevated prior to initiating AZMIRO. Periodically monitor hematocrit levels during treatment. If hematocrit becomes elevated, stop AZMIRO until hematocrit decreases to an acceptable concentration. If AZMIRO is restarted and again causes hematocrit to become elevated, stop AZMIRO permanently. An increase in red blood cell mass may increase the risk of thromboembolic events [ see Warnings and Precautions ( 5.2) ].
5.2 Venous Thromboembolism (VTE)
There have been postmarketing reports of venous thromboembolic events, including deep vein thrombosis (DVT) and pulmonary embolism (PE), in patients using testosterone replacement products, such as AZMIRO.
In the Testosterone Replacement therapy for Assessment of long-term Vascular Events and efficacy ResponSE in hypogonadal men (TRAVERSE) Study, a randomized, double-blind, placebo controlled, cardiovascular (CV) outcomes study, compared to placebo, topical testosterone gel was associated with a numerically higher incidence of VTE (1.7% vs 1.2%) which included DVT (0.6% vs 0.5%) and PE events (0.9% vs 0.5%) [see Adverse Reactions (6.1)].
Evaluate patients who report symptoms of pain, edema, warmth and erythema in the lower extremity for DVT and those who present with acute shortness of breath for PE. If a venous thromboembolic event is suspected, discontinue treatment with AZMIRO and initiate appropriate workup and management [see Adverse Reactions (6.2)].5.3 Worsening of Benign Prostatic Hyperplasia (BPH) and Potential Risk of Prostate Cancer
Patients with BPH treated with androgens are at an increased risk for worsening of signs and symptoms of BPH. Monitor patients with BPH for worsening signs and symptoms.
Patients treated with androgens may be at increased risk for prostate cancer. Evaluate patients for prostate cancer prior to initiating and during treatment with androgens [ see Contraindications ( 4) ].5.4 Blood Pressure Increases
Testosterone products, such as AZMIRO, can increase blood pressure. Blood pressure increases can increase cardiovascular (CV) risk over time.
The CV risk associated with topical testosterone gel was evaluated in TRAVERSE, randomized, double-blind, placebo-controlled, CV outcomes study in men with a history of CV disease or multiple CV risk factors. In TRAVERSE, topical testosterone gel increased mean systolic blood pressure by 1.0 mm Hg from baseline to 36 months, whereas a mean decrease from baseline of 0.5 mm Hg was observed in the placebo group at this timepoint, for a mean between-group difference of 1.5 mm Hg. However, the incidences of major adverse cardiovascular events (MACE), including cardiovascular death, non-fatal myocardial infarction [MI] and non-fatal stroke, were similar between treatment groups (7% for topical testosterone gel vs 7.3% for placebo) [See Adverse Reactions (6.1)].
Monitor blood pressure periodically in men using AZMIRO, especially men with hypertension. AZMIRO is not recommended for use in patients with uncontrolled hypertension.5.5 Abuse of Testosterone and Monitoring of Serum Testosterone Concentrations
Testosterone has been subject to abuse, typically at doses higher than recommended for the approved indication and in combination with other anabolic androgenic steroids. Anabolic androgenic steroid abuse can lead to serious cardiovascular and psychiatric adverse reactions [ see Drug Abuse And Dependence ( 9) ].
If testosterone abuse is suspected, check serum testosterone concentrations to ensure they are within therapeutic range. However, testosterone levels may be in the normal or subnormal range in men abusing synthetic testosterone derivatives. Counsel patients concerning the serious adverse reactions associated with abuse of testosterone and anabolic androgenic steroids. Conversely, consider the possibility of testosterone and anabolic androgenic steroid abuse in suspected patients who present with serious cardiovascular or psychiatric adverse events.5.6 Not for Use in Women
Due to lack of controlled studies in women and the potential for virilizing effects, AZMIRO is not indicated for use in women [ see Use in Specific Populations ( 8.1, 8.2) ].
5.7 Potential for Adverse Effects on Spermatogenesis
With large doses of exogenous androgens, including AZMIRO, spermatogenesis may be suppressed through feedback inhibition of pituitary follicle-stimulating hormone (FSH) possibly leading to adverse effects on semen parameters including sperm count [ see Use in Specific Populations ( 8.3) and Adverse Reactions ( 6.2) ]. Patients should be informed of this possible risk when deciding whether to use or to continue to use AZMIRO.
5.8 Hepatic Adverse Effects
Prolonged use of high doses of orally active 17-alpha-alkyl androgens (e.g., methyltestosterone) has been associated with serious hepatic adverse effects (peliosis hepatis, hepatic neoplasms, cholestatic hepatitis, and jaundice). Peliosis hepatis can be a life-threatening or fatal complication. Long-term therapy with intramuscular testosterone enanthate has produced multiple hepatic adenomas. AZMIRO is not a 17 alpha-alkyl androgen and is not known to produce hepatic adverse effects associated with 17-alpha-alkyl androgens.
Nonetheless, patients should be instructed to report any signs or symptoms of hepatic dysfunction (e.g., jaundice). If these occur, promptly discontinue AZMIRO while the cause is evaluated.5.9 Edema
Androgens, including AZMIRO, may promote retention of sodium and water. Edema, with or without congestive heart failure, may be a serious complication in patients with pre-existing cardiac, renal or hepatic disease [ see Adverse Reactions ( 6.2) ]. In addition to discontinuation of the drug, appropriate work up and management of edema may be required.
5.10 Sleep Apnea
The treatment of hypogonadal men with testosterone products may potentiate sleep apnea in some patients, especially those with risk factors such as obesity or chronic lung diseases.
5.11 Gynecomastia
Gynecomastia may develop and occasionally persists in patients being treated for hypogonadism.
5.12 Lipid Changes
Changes in serum lipid profile may require dose adjustment of lipid lowering drugs or discontinuation of testosterone therapy. Monitor the lipid profile periodically after starting testosterone therapy.
5.13 Hypercalcemia
Androgens, including AZMIRO, should be used with caution in cancer patients at risk of hypercalcemia (and associated hypercalciuria). Monitor serum calcium concentrations periodically in these patients.
5.14 Decreased Thyroxine-binding Globulin
Androgens, including AZMIRO, may decrease concentrations of thyroxine-binding globulin, resulting in decreased total T 4 serum levels and increased resin uptake of T 3 and T 4. Free thyroid hormone levels remain unchanged, however, and there is no clinical evidence of thyroid dysfunction.
5.15 Increases in Prolactin
Increases in serum prolactin have been reported in patients treated with testosterone products, such as AZMIRO. Evaluate serum prolactin levels prior to initiating treatment with AZMIRO. Re-evaluate serum prolactin levels 3 to 4 months after starting treatment. If serum prolactin remains elevated, discontinue AZMIRO.
5.16 Adverse Effects on Bone Maturation
Testosterone use may result in acceleration of bone age and premature closure of epiphyses in pediatric patients. This adverse effect may result in compromised adult stature. The younger the pediatric patient the greater the risk of compromising final mature height. Monitor the effect on bone maturation by assessing bone age of the wrist and hand every 6 months.
-
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are discussed elsewhere in the labeling:
Polycythemia [ see Warnings and Precautions ( 5.1) ]
Venous Thromboembolism [ see Warnings and Precautions ( 5.2)]
Worsening of Benign Prostatic Hyperplasia (BPH) and Potential Risk of Prostate Cancer [ see Warnings and Precautions ( 5.3) ]
Blood Pressure Increases [ see Warnings and Precautions ( 5.4) ]
Hepatic Adverse Effects [ see Warnings and Precautions ( 5.8) ]
Edema [ see Warnings and Precautions ( 5.9) ]
Sleep Apnea [ see Warnings and Precautions ( 5.10) ]
Gynecomastia [ see Warnings and Precautions ( 5.11) ]
Lipid Changes [ see Warnings and Precautions ( 5.12) ]
Hypercalcemia [ see Warnings and Precautions ( 5.13) ]
Decreased Thyroxine-binding Globulin [ see Warnings and Precautions ( 5.14) ]
Increases in Prolactin [ see Warnings and Precautions ( 5.15) ]
Adverse Effects on Bone Maturation [ see Warnings and Precautions ( 5.16) ]6.1 Clinical Trial Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of AZMIRO was evaluated, in Study 1, a randomized, single-dose, open-label study conducted in 27 adult males with hypogonadism. Patients were 18 to 65 years of age with a body mass index of 18 to 35 kg/m2. Patients received a single intramuscular dose AZMIRO 200 mg or comparator intramuscular testosterone replacement therapy product and were observed for adverse reactions and injection site reactions over 31 days.
The most common adverse reactions in patients who received AZMIRO were injection site erythema (26%) and injection site reaction (4%). All cases of injection site erythema and injection site reaction were categorized as mild based on a pre-defined injection site assessment scale that defined injection site reactions as mild if they were slight or barely perceptible.Cardiovascular Outcomes
TRAVERSE was a randomized, double-blind, cardiovascular outcomes study to assess the cardiovascular (CV) safety of topical testosterone gel compared to placebo in 5198 hypogonadal men aged 45 to 80 years with a history of CV disease or with multiple CV risk factors. The primary outcome was the incidence of the composite endpoint of major adverse cardiovascular events (MACE), consisting of CV death, non-fatal myocardial infarction (MI), and non-fatal stroke The mean duration of therapy was approximately 22 months. The mean duration of follow-up was 33 months. Approximately 61% of all patients discontinued topical testosterone gel or placebo therapy.
The mean patient age (±SD) was 63.3 (7.9) years, with 2452 patients aged 65 years or more (47%); 2847 (about 55%) patients had pre-existing cardiovascular disease, whereas 2357 patients (about 45%) had an elevated cardiovascular risk at baseline, and mean BMI was 35 kg/m2. Approximately 80% of patients were White, 17% were Black, and 3% were of other races or ethnic groups. Approximately 69%, 84%, and 93% had diabetes mellitus, hyperlipidemia, and hypertension, respectively.
The mean serum testosterone concentration at baseline in patients receiving topical testosterone gel was 220.4 ng/dL (n=2596). The mean serum testosterone concentrations at 12 months, 24 months, 36 months, and 48 months in patients receiving topical testosterone gel were 440.5 ng/dL (n=1683), 420.9 ng/dl (n=1125), 428.7 ng/dL (n=731), and 365.2 ng/dL (n=220), respectively.
For patients treated with topical testosterone gel, the incidence of MACE was 7.0% (n=182 events) and for those receiving placebo, the incidence of MACE was 7.3% (n=190 events). The study demonstrated non-inferiority of topical testosterone gel versus placebo because the upper bound of 95% CI was less than the pre-specified risk margin, of 1.5 for MACE (Hazard Ratio 0.96 [95% CI: 0.78, 1.17]).
Additional Adverse Reactions Reported in TRAVERSE
Additional adverse reactions reported in TRAVERSE at an incidence rate >2% in either treatment group and greater in topical testosterone gel versus placebo included: nonfatal arrythmias warranting intervention (5.2% vs 3.3%), atrial fibrillation (3.5% vs 2.4%), acute kidney injury (2.3% vs 1.5%) and bone fracture (3.5% vs 2.5%). For the adverse reaction of bone fracture, each event was adjudicated by clinical review.6.2 Other Adverse Reactions
The following adverse reactions associated with the use of testosterone were identified in clinical studies or postmarketing reports. Because some of these reactions were reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Administration site reactions: Inflammation and pain at the site of intramuscular injection.
Allergic: Hypersensitivity, including skin manifestations and anaphylactoid reactions.
Cardiovascular disorders: myocardial infarction, stroke.
Endocrine and urogenital: Gynecomastia, premature closure of bony epiphyses with termination of growth, precocious puberty.
Fluid and electrolyte disturbances: Retention of sodium, chloride, water, potassium, calcium, and inorganic phosphates.
Gastrointestinal: Nausea, cholestatic jaundice, alterations in liver function tests, rarely hepatocellular neoplasms and peliosis hepatis.
Hematologic: Suppression of clotting factors II, V, VII, and X, bleeding in patients on concomitant anticoagulant therapy, and polycythemia.
Nervous system: Increased or decreased libido, headache, anxiety, depression, and generalized paresthesia.
Reproductive system: Excessive frequency and duration of penile erections, oligospermia, and priapismVascular disorders: Venous thromboembolism.
Skin and appendages: Male pattern baldness, seborrhea, and acne.
Special senses: Rare cases of central serous chorioretinopathy (CSCR). -
7 DRUG INTERACTIONS
7.1 Insulin
Changes in insulin sensitivity or glycemic control may occur in patients treated with androgens. In diabetic patients, the metabolic effects of androgens may decrease blood glucose and, therefore, insulin requirements.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
AZMIRO is contraindicated in pregnant women and not indicated for use in females [see Contraindications ( 4)]. Testosterone is teratogenic and may cause fetal harm when administered to a pregnant woman based on data from animal studies ( see Data) and its mechanism of action [ see Clinical Pharmacology ( 12.1) ]. Exposure of a female fetus to androgens may result in varying degrees of virilization. In animal developmental studies, exposure to testosterone in utero resulted in hormonal and behavioral changes in offspring and structural impairments of reproductive tissues in female and male offspring. These studies did not meet current standards for nonclinical development toxicity studies.
Data
Animal Data
In developmental studies conducted in rats, rabbits, pigs, sheep and rhesus monkeys, pregnant animals received intramuscular injection of testosterone during the period of organogenesis. Testosterone treatment at doses that were comparable to those used for testosterone replacement therapy resulted in structural impairments in both female and male offspring. Structural impairments observed in females included increased anogenital distance, phallus development, empty scrotum, no external vagina, intrauterine growth retardation, reduced ovarian reserve, and increased ovarian follicular recruitment. Structural impairments seen in male offspring included increased testicular weight, larger seminal tubular lumen diameter, and higher frequency of occluded tubule lumen. Increased pituitary weight was seen in both sexes.
Testosterone exposure in utero also resulted in hormonal and behavioral changes in offspring. Hypertension was observed in pregnant females and offspring in rats exposed to doses approximately twice those used for testosterone replacement therapy.8.3 Females and Males of Reproductive Potential
Infertility
Males
During treatment with large doses of exogenous androgens, including AZMIRO, spermatogenesis may be suppressed through feedback inhibition of the hypothalamic-pituitary-testicular-axis [ see Warnings and Precautions ( 5.7) ]. Reduced fertility is observed in some men taking testosterone replacement therapy. The impact on fertility may be irreversible. Testicular atrophy, subfertility, and infertility have also been reported in men who abuse anabolic androgenic steroids [ see Drug Abuse and Dependence ( 9.2) ].8.4 Pediatric Use
Improper use may result in acceleration of bone age and premature closure of epiphyses. The effect on bone maturation should be monitored by assessing bone age of the wrist and hand every 6 months. In children, androgen treatment may accelerate bone maturation without producing compensatory gain in linear growth. This adverse effect may result in compromised adult stature. The younger the child the greater the risk of compromising final mature height. Precocious puberty has also been reported with use of testosterone.
The safety and effectiveness of AZMIRO have not been established in pediatric patients young than 12 years of age.8.5 Geriatric Use
Geriatric patients treated with androgens may be at an increased risk of developing BPH and prostatic carcinoma [see Warnings and Precautions ( 5.3)].
-
9 DRUG ABUSE AND DEPENDENCE
9.2 Abuse
Drug abuse is intentional non-therapeutic use of a drug, even once, for its rewarding psychological and physiological effects. Abuse and misuse of testosterone are seen in male and female adults and adolescents. Testosterone, often in combination with other anabolic androgenic steroids (AAS), and not obtained by prescription through a pharmacy, may be abused by athletes and bodybuilders. There have been reports of misuse by men taking higher doses of legally obtained testosterone than prescribed and continuing testosterone despite adverse events or against medical advice.
Abuse-Related Adverse Reactions
Serious adverse reactions have been reported in individuals who abuse anabolic androgenic steroids, and include cardiac arrest, myocardial infarction, hypertrophic cardiomyopathy, congestive heart failure, cerebrovascular accident, hepatotoxicity, and serious psychiatric manifestations, including major depression, mania, paranoia, psychosis, delusions, hallucinations, hostility, and aggression.
The following adverse reactions have also been reported in men: transient ischemic attacks, convulsions, hypomania, irritability, dyslipidemias, testicular atrophy, subfertility, and infertility.
The following additional adverse reactions have been reported in women: hirsutism, virilization, deepening of voice, clitoral enlargement, breast atrophy, male-pattern baldness, and menstrual irregularities.
The following adverse reactions have been reported in male and female adolescents: premature closure of bony epiphyses with termination of growth, and precocious puberty.
Because these reactions are reported voluntarily from a population of uncertain size and may include abuse of other agents, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.9.3 Dependence
Behaviors Associated with Addiction
Continued abuse of testosterone and other anabolic steroids, leading to addiction is characterized by the following behaviors:
Taking greater dosages than prescribed
Continued drug use despite medical and social problems due to drug use
Spending significant time to obtain the drug when supplies of the drug are interrupted
Giving a higher priority to drug use than other obligations
Having difficulty in discontinuing the drug despite desires and attempts to do so
Experiencing withdrawal symptoms upon abrupt discontinuation of use
Physical dependence is characterized by withdrawal symptoms after abrupt drug discontinuation or a significant dose reduction of a drug. Individuals taking supratherapeutic doses of testosterone may experience withdrawal symptoms lasting for weeks or months which include depressed mood, major depression, fatigue, craving, restlessness, irritability, anorexia, insomnia, decreased libido and hypogonadotropic hypogonadism.
Drug dependence in individuals using approved doses of testosterone for approved indications has not been documented. -
10 OVERDOSAGE
There is one report of acute overdosage with use of an approved injectable testosterone product: this subject had serum testosterone levels of up to 11,400 ng/dL with a cerebrovascular accident. Treatment of overdosage consists of discontinuation of AZMIRO and appropriate symptomatic and supportive care.
-
11 DESCRIPTION
AZMIRO (testosterone cypionate) injection for intramuscular injection, contains testosterone cypionate which is the oil-soluble 17 (beta)-cyclopentylpropionate ester of the androgenic hormone testosterone.
Testosterone cypionate is a white or creamy white crystalline powder, odorless or nearly so and stable in air. It is insoluble in water, freely soluble in alcohol, chloroform, dioxane, ether, and soluble in vegetable oils.
The chemical name for testosterone cypionate is androst-4-en-3-one, 17-(3-cyclopentyl-1-oxopropoxy)-, (17ß)-. Its molecular formula is C 27H 40O 3, and the molecular weight 412.61. The structural formula is shown in the following figure:
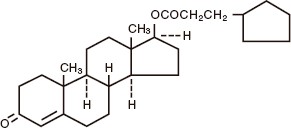
AZMIRO (testosterone cypionate) injection is provided as sterile, clear colorless to pale yellow solution containing 200 mg/mL testosterone cypionate in vials and prefilled syringes.
Each mL of solution contains:
Testosterone cypionate………………………………………..200 mg
Benzyl alcohol………………………………………………….20 mg
Benzyl benzoate……………………………………………….0.2 mL
Cottonseed oil…………………………………………………542 mg -
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Endogenous androgens, including testosterone and dihydrotestosterone (DHT), are responsible for normal growth and development of the male sex organs and for maintenance of secondary sex characteristics. These effects include growth and maturation of the prostate, seminal vesicles, penis, and scrotum; the development of male hair distribution, such as facial, pubic, chest, and axillary hair; laryngeal enlargement, vocal cord thickening, alterations in body musculature and fat distribution.
Male hypogonadism, a clinical syndrome resulting from insufficient secretion of testosterone, has two main etiologies. Primary hypogonadism is caused by defects of the gonads, such as Klinefelter's Syndrome or Leydig cell aplasia, whereas secondary hypogonadism is the failure of the hypothalamus (or pituitary) to produce sufficient gonadotropins (FSH, LH).12.2 Pharmacodynamics
The exposure-response relationship and time course of pharmacodynamic response for the safety and effectiveness of testosterone have not been fully characterized.
12.3 Pharmacokinetics
Absorption
Testosterone esters are less polar than free testosterone. Testosterone esters in oil injected intramuscularly are absorbed slowly from the lipid phase; thus, AZMIRO can be given at intervals of two to four weeks.
Pharmacokinetic parameters of baseline-corrected testosterone obtained following a single-dose intramuscular administration of AZMIRO 200 mg in prefilled syringe in 26 hypogonadal adult males are summarized in Table 1 below.
Table 1: Mean (±SD) Baseline-corrected Testosterone Pharmacokinetic Parameters
Following a Single-dose Intramuscular Administration of AZMIRO 200 mg in Hypogonadal Males (N=26)
Parameter
Mean (±SD)
AUC 0-t(hr·ng/dL)
137218.7 (±62360.0)
C max(ng/dL)
758.0 (±288.7)
T max(hr)
71.7 (24.0, 191.0) a
aReported in median (min, max).
Distribution
Circulating testosterone is primarily bound in serum to sex hormone-binding globulin (SHBG) and albumin. Approximately 40% of testosterone in plasma is bound to SHBG, 2% remains unbound (free) and the rest is bound to albumin and other proteins.
Elimination
The half-life of AZMIRO when injected intramuscularly is approximately eight days.
Metabolism
Inactivation of testosterone occurs primarily in the liver. Testosterone is metabolized to various 17-keto steroids through two different pathways. The major active metabolites of testosterone are dihydrotestosterone (DHT) and estradiol.
Excretion
About 90 percent of a dose of testosterone is excreted in the urine as glucuronic and sulfuric acid conjugates of testosterone and its metabolites. About 6 percent of a dose is excreted in the feces, mostly in the unconjugated form. -
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Testosterone has been tested by subcutaneous injection and implantation in mice and rats. The implant induced cervical-uterine tumors in mice, which metastasized in some cases.
There is suggestive evidence that injection of testosterone into some strains of female mice increases their susceptibility to hepatoma. Testosterone is also known to increase the number of tumors and decrease the degree of differentiation of chemically induced carcinomas of the liver in rats.
Mutagenicity
Testosterone was negative in the in vitro Ames and in the in vivo mouse micronucleus assays.
Impairment of Fertility
The administration of exogenous testosterone suppresses spermatogenesis in the rat, dog and non-human primates, which was reversible on cessation of the treatment. -
16 HOW SUPPLIED/STORAGE AND HANDLING
AZMIRO (testosterone cypionate) injection is supplied as a sterile, clear colorless to pale yellow solution in single-dose vials and single-dose prefilled syringes as 200 mg/mL testosterone cypionate.
NDC Number
Package Size
24338-056-01
1 mL vials
24338-055-01
1 mL prefilled syringes Store at 15°C to 25°C (59°F to 77°F); excursions permitted to 2°C to 30°C (36°F to 86°F). Store product in carton to protect contents from light.
-
17 PATIENT COUNSELING INFORMATION
Polycythemia
Advise patients that AZMIRO can cause an increase in hematocrit levels that may increase the risk of thromboembolic events. Advise patients about the importance of completing laboratory testing as instructed by their health care provider while on AZMIRO [ see Warnings and Precautions ( 5.1) ].Venous Thromboembolism
Advise patients that AZMIRO can cause venous thromboembolism. Advise patients of the signs and symptoms of venous thromboembolism, which may include the following: lower limb pain, edema, or erythema; and dyspnea or chest pain. Advise patients to promptly report the signs and symptoms of venous thromboembolism, discontinue use of AZMIRO, and seek urgent medical care.Increase in Blood Pressure
Advise patients that AZMIRO can increase BP which can increase cardiovascular risk over time. Instruct patients about the importance of monitoring BP periodically while on AZMIRO. If BP increases while on AZMIRO, antihypertensive medications may need to be started, added, or adjusted to control BP, or AZMIRO may need to be discontinued.Worsening of Benign Prostatic Hyperplasia (BPH) and Potential Risk of Prostate Cancer
Advise patients that AZMIRO can cause increased symptoms of BPH and can increase the risk for prostate cancer. Advise patients to contact their health care provider if they have any prostate-related symptoms [ see Warnings and Precautions ( 5.3) ].
Edema
Advise patients with preexisting cardiac, renal, or hepatic disease that AZMIRO can cause edema. Advise patients to notify their health care provider if edema develops or worsens [ see Warnings and Precautions ( 5.9) ].
Sleep Apnea
Advise patients that AZMIRO can worsen sleep apnea especially in patients with risk factors such as obesity or chronic lung diseases [ see Warnings and Precautions ( 5.10) ].
Gynecomastia
Advise patients that AZMIRO can cause gynecomastia [ see Warnings and Precautions ( 5.11) ].
Manufactured for:
Azurity Pharmaceuticals, Inc.
Woburn, MA 01801
Manufactured by:
LSNE-LEON SLU
Calle nicostrato vela
(pq. Tecchnologico leon),
S/N – M1.1-M1.2, Leon, 24009,
Spain (ESP)
Patent: https://azurity.com/patents_and_trademarks/
This product's labeling may have been updated. For current Full Prescribing Information, please visit www.azmiro.com
AZM-PI-01 Rev. JULY2025 -
MEDICATION GUIDE
MEDICATION GUIDE
AZMIRO TM (AZ-MEER-OH)
(testosterone cypionate)
injection, for intramuscular use, CIII
What is the most important information I should know about AZMIRO?
AZMIRO can cause serious side effects, including:
Increase in red blood cell counts (hematocrit) or hemoglobin.
o AZMIRO increases red blood cell counts in some people. High red blood cell counts increase the risk of blood clots, strokes, and heart attacks.
o You may need to stop AZMIRO if your red blood cell count increases.
o Your healthcare provider should check your red blood cell count and hemoglobin while you receive AZMIRO.What is AZMIRO?
AZMIRO is a prescription medicine that contains testosterone. AZMIRO is used to treat adult men who have low or no testosterone due to certain medical conditions.
It is not known if AZMIRO is safe or effective to treat men who have low testosterone due to aging.
It is not known if AZMIRO is safe or effective in children younger than 12 years old. Improper use of testosterone may affect bone growth in children.
AZMIRO is a controlled substance (CIII) because it contains testosterone that can be a target for people who abuse prescription medicines. Selling or giving away this medicine may harm others and it is against the law.
AZMIRO is not meant for use in women.Do not receive AZMIRO if you:
are allergic to AZMIRO or to any ingredients in AZMIRO. See the end of this Medication Guide for a complete list of ingredients in AZMIRO.
have breast cancer.
have or might have prostate cancer.
are a woman who is pregnant. AZMIRO may harm your unborn baby.What should I tell my healthcare provider before receiving AZMIRO?
Before receiving AZMIRO, tell your healthcare provider about all of your medical conditions including if you:
have high red blood cell count (hematocrit) or high hemoglobin laboratory value.
have breast cancer.
have or might have prostate cancer.
have urinary problems due to an enlarged prostate.
have high blood pressure or are being treated for high blood pressure.
have heart problems.
have liver or kidney problems.
have problems breathing while you sleep (sleep apnea).
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Using AZMIRO with certain other medicines can affect each other. Especially, tell your healthcare provider if you take:
insulin.
medicines that decrease blood clotting (blood thinners).
corticosteroids.
Ask your healthcare provider or pharmacist for a list of these medicines, if you are not sure. Know the medicines you take. Keep a list of your medicines and show them to your healthcare provider and pharmacist when you get a new medicine.
How will I receive AZMIRO?
Your healthcare provider will inject AZMIRO deep into the muscle of your buttock.
Your healthcare provider will test your blood before you receive and while you are receiving AZMIRO.What are the possible side effects of AZMIRO?
AZMIRO may cause serious side effects including:
See “What is the most important information I should know about AZMIRO?”
If you already have an enlarged prostate, your signs and symptoms may worsen while receiving AZMIRO. This may include:
o increased urination at night.
o trouble starting your urine stream.
o urinating many times during the day.
o urge to go to the bathroom right away.
o a urine accident.
o inability to pass urine or weak urine flow.
Increased risk of prostate cancer. Your healthcare provider should check you for prostate cancer or any other prostate problems before you start and while you receive AZMIRO.
Blood clots in the legs or lungs. Signs and symptoms of a blood clot in your leg can include leg pain, swelling or redness. Signs and symptoms of a blood clot in your lungs can include difficulty breathing or chest pain.
Increased blood pressure. AZMIRO can increase your blood pressure, which can increase your risk of having a heart attack or stroke over time. Your risk may be greater if you have already had a heart attack or stroke or if you have other risk factors for heart attack or stroke. You may need to start new medicines or have medicines changed for high blood pressure while on AZMIRO. If your blood pressure cannot be controlled, AZMIRO may need to be stopped. Your healthcare provider should check your blood pressure while you use AZMIRO.
Abuse. Testosterone can be abused, when taken at higher than prescribed doses and when used with other anabolic steroids. Abuse can cause serious heart and psychological side effects. Your healthcare provider should check you for signs of abuse before and during treatment with AZMIRO.
AZMIRO may lower your sperm count.
Liver problems. Symptoms of liver problems may include:
o nausea or vomiting.
o yellowing of your skin or whites of your eyes.
o dark urine.
o pain on the right side of your stomach area (abdominal pain).
Swelling of your ankles, feet, or body (edema), with or without heart failure. This may cause serious problems for people who have heart, kidney or liver disease.
Breathing problems while you sleep (sleep apnea).
Enlarged or painful breasts.
Changes in certain blood tests. These changes include changes in liver tests, increased calcium, decreased thyroid hormones, and increased prolactin hormones (hormone made by pituitary gland) levels.
Increased risk in children of bone growth problems. AZMIRO may affect a child’s final adult height (stature). The child’s bone growth should be checked every 6 months while they are receiving AZMIRO.
Call your healthcare provider right away if you have any of the serious side effects listed above.
The most common side effects of AZMIRO include:
injection site reactions including: bruising, bleeding, redness, hardness.
increased red blood cell count.
enlarged or painful breasts.
headache.
depression.
Other side effects include more erections than are normal for you or erections that last a long time. Tell your healthcare provider if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of AZMIRO. For more information, ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of AZMIRO.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. You can ask your pharmacist or healthcare provider for information about AZMIRO that is written for healthcare professionals.What are the ingredients in AZMIRO?
Active ingredient: testosterone
Inactive ingredients: benzyl alcohol, benzyl benzoate and cottonseed oil.
Manufactured for:
Azurity Pharmaceuticals, Inc.
Woburn, MA 01801
Manufactured by:
LSNE-LEON SLU
Calle nicostrato vela
(pq. Tecchnologico leon),
S/N – M1.1-M1.2, Leon, 24009,
Spain (ESP)
Patent: https://azurity.com/patents_and_trademarks/
This Medication Guide may have been updated. For current Medication Guide Information, please visit www.azmiro.com
AZM-MG-01 Rev. JULY2025
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
200 MG/ML - VIAL LABEL
NDC: 24338-056-01
1 mL Single-Dose Vial
AZMIRO (testosterone cypionate) injection, USP
CIIIFor intramuscular use only
Rx only

Principal Display Panel - 200 MG/ML - VIAL CARTON
NDC: 24338-056-01
1 mL Single-Dose Vial
AZMIRO (testosterone cypionate) injection, USP
CIII
For intramuscular use only
Rx only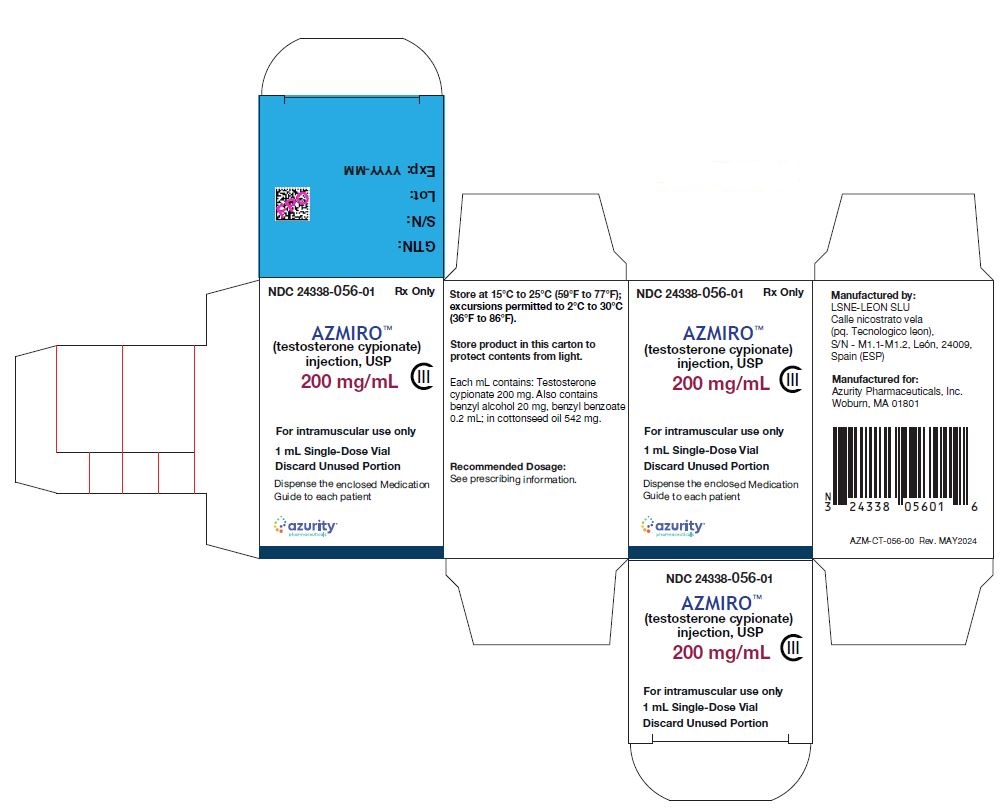
200 MG/ML - PREFILLED SYRINGE LABEL
NDC: 24338-055-01
1 mL Single-dose Prefilled Syringe
AZMIRO (testosterone cypionate) injection, USP
CIII
For intramuscular use only
Rx only
Principal Display Panel - 200 MG/ML - PREFILLED SYRINGE CARTON
NDC: 24338-055-01
1 mL Single-dose Prefilled Syringe
AZMIRO (testosterone cypionate) injection, USP
CIII
For intramuscular use only
Rx only
-
INGREDIENTS AND APPEARANCE
AZMIRO
testosterone cypionate injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 24338-056 Route of Administration INTRAMUSCULAR DEA Schedule CIII Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TESTOSTERONE CYPIONATE (UNII: M0XW1UBI14) (TESTOSTERONE - UNII:3XMK78S47O) TESTOSTERONE CYPIONATE 200 mg in 1 mL Inactive Ingredients Ingredient Name Strength BENZYL BENZOATE (UNII: N863NB338G) 0.2 mL in 1 mL COTTONSEED OIL (UNII: H3E878020N) 542 mg in 1 mL BENZYL ALCOHOL (UNII: LKG8494WBH) 20 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 24338-056-01 1 in 1 CARTON 10/04/2024 1 1 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA216318 10/04/2024 AZMIRO
testosterone cypionate injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 24338-055 Route of Administration INTRAMUSCULAR DEA Schedule CIII Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TESTOSTERONE CYPIONATE (UNII: M0XW1UBI14) (TESTOSTERONE - UNII:3XMK78S47O) TESTOSTERONE CYPIONATE 200 mg in 1 mL Inactive Ingredients Ingredient Name Strength BENZYL BENZOATE (UNII: N863NB338G) 0.2 mL in 1 mL COTTONSEED OIL (UNII: H3E878020N) 542 mg in 1 mL BENZYL ALCOHOL (UNII: LKG8494WBH) 20 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 24338-055-01 1 in 1 CARTON 10/04/2024 1 1 mL in 1 SYRINGE, GLASS; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA216318 10/04/2024 Labeler - Azurity Pharmaceuticals, Inc. (117505635)
Trademark Results [AZMIRO]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 AZMIRO 98275054 not registered Live/Pending |
Azurity Pharmaceuticals, Inc. 2023-11-17 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.