CELLCEPT- mycophenolate mofetil capsule
CellCept by
Drug Labeling and Warnings
CellCept by is a Prescription medication manufactured, distributed, or labeled by Rebel Distributors Corp. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
BOXED WARNING
(What is this?)
Immunosuppression may lead to increased susceptibility to infection and possible development of lymphoma. Only physicians experienced in immunosuppressive therapy and management of renal, cardiac or hepatic transplant patients should use CellCept. Patients receiving the drug should be managed in facilities equipped and staffed with adequate laboratory and supportive medical resources. The physician responsible for maintenance therapy should have complete information requisite for the follow-up of the patient.
Female users of childbearing potential must use contraception. Use of CellCept during pregnancy is associated with increased risk of pregnancy loss and congenital malformations.
-
DESCRIPTION
CellCept (mycophenolate mofetil) is the 2-morpholinoethyl ester of mycophenolic acid (MPA), an immunosuppressive agent; inosine monophosphate dehydrogenase (IMPDH) inhibitor.
The chemical name for mycophenolate mofetil (MMF) is 2-morpholinoethyl (E)-6-(1,3-dihydro-4-hydroxy-6-methoxy-7-methyl-3-oxo-5-isobenzofuranyl)-4-methyl-4-hexenoate. It has an empirical formula of C23H31NO7, a molecular weight of 433.50, and the following structural formula:
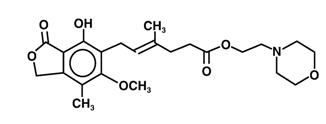
Mycophenolate mofetil is a white to off-white crystalline powder. It is slightly soluble in water (43 µg/mL at pH 7.4); the solubility increases in acidic medium (4.27 mg/mL at pH 3.6). It is freely soluble in acetone, soluble in methanol, and sparingly soluble in ethanol. The apparent partition coefficient in 1-octanol/water (pH 7.4) buffer solution is 238. The pKa values for mycophenolate mofetil are 5.6 for the morpholino group and 8.5 for the phenolic group.
Mycophenolate mofetil hydrochloride has a solubility of 65.8 mg/mL in 5% Dextrose Injection USP (D5W). The pH of the reconstituted solution is 2.4 to 4.1.
CellCept is available for oral administration as capsules containing 250 mg of mycophenolate mofetil, tablets containing 500 mg of mycophenolate mofetil, and as a powder for oral suspension, which when constituted contains 200 mg/mL mycophenolate mofetil.
Inactive ingredients in CellCept 250 mg capsules include croscarmellose sodium, magnesium stearate, povidone (K-90) and pregelatinized starch. The capsule shells contain black iron oxide, FD&C blue #2, gelatin, red iron oxide, silicon dioxide, sodium lauryl sulfate, titanium dioxide, and yellow iron oxide.
Inactive ingredients in CellCept 500 mg tablets include black iron oxide, croscarmellose sodium, FD&C blue #2 aluminum lake, hydroxypropyl cellulose, hydroxypropyl methylcellulose, magnesium stearate, microcrystalline cellulose, polyethylene glycol 400, povidone (K-90), red iron oxide, talc, and titanium dioxide; may also contain ammonium hydroxide, ethyl alcohol, methyl alcohol, n-butyl alcohol, propylene glycol, and shellac.
Inactive ingredients in CellCept Oral Suspension include aspartame, citric acid anhydrous, colloidal silicon dioxide, methylparaben, mixed fruit flavor, sodium citrate dihydrate, sorbitol, soybean lecithin, and xanthan gum.
CellCept Intravenous is the hydrochloride salt of mycophenolate mofetil. The chemical name for the hydrochloride salt of mycophenolate mofetil is 2-morpholinoethyl (E)-6-(1,3-dihydro-4-hydroxy-6-methoxy-7-methyl-3-oxo-5-isobenzofuranyl)-4-methyl-4-hexenoate hydrochloride. It has an empirical formula of C23H31NO7 HCl and a molecular weight of 469.96.
CellCept Intravenous is available as a sterile white to off-white lyophilized powder in vials containing mycophenolate mofetil hydrochloride for administration by intravenous infusion only. Each vial of CellCept Intravenous contains the equivalent of 500 mg mycophenolate mofetil as the hydrochloride salt. The inactive ingredients are polysorbate 80, 25 mg, and citric acid, 5 mg. Sodium hydroxide may have been used in the manufacture of CellCept Intravenous to adjust the pH. Reconstitution and dilution with 5% Dextrose Injection USP yields a slightly yellow solution of mycophenolate mofetil, 6 mg/mL. (For detailed method of preparation, see DOSAGE AND ADMINISTRATION).
-
CLINICAL PHARMACOLOGY
Mechanism of Action
Mycophenolate mofetil has been demonstrated in experimental animal models to prolong the survival of allogeneic transplants (kidney, heart, liver, intestine, limb, small bowel, pancreatic islets, and bone marrow).
Mycophenolate mofetil has also been shown to reverse ongoing acute rejection in the canine renal and rat cardiac allograft models. Mycophenolate mofetil also inhibited proliferative arteriopathy in experimental models of aortic and cardiac allografts in rats, as well as in primate cardiac xenografts. Mycophenolate mofetil was used alone or in combination with other immunosuppressive agents in these studies. Mycophenolate mofetil has been demonstrated to inhibit immunologically mediated inflammatory responses in animal models and to inhibit tumor development and prolong survival in murine tumor transplant models.
Mycophenolate mofetil is rapidly absorbed following oral administration and hydrolyzed to form MPA, which is the active metabolite. MPA is a potent, selective, uncompetitive, and reversible inhibitor of inosine monophosphate dehydrogenase (IMPDH), and therefore inhibits the de novo pathway of guanosine nucleotide synthesis without incorporation into DNA. Because T- and B-lymphocytes are critically dependent for their proliferation on de novo synthesis of purines, whereas other cell types can utilize salvage pathways, MPA has potent cytostatic effects on lymphocytes. MPA inhibits proliferative responses of T- and B-lymphocytes to both mitogenic and allospecific stimulation. Addition of guanosine or deoxyguanosine reverses the cytostatic effects of MPA on lymphocytes. MPA also suppresses antibody formation by B-lymphocytes. MPA prevents the glycosylation of lymphocyte and monocyte glycoproteins that are involved in intercellular adhesion to endothelial cells and may inhibit recruitment of leukocytes into sites of inflammation and graft rejection. Mycophenolate mofetil did not inhibit early events in the activation of human peripheral blood mononuclear cells, such as the production of interleukin-1 (IL-1) and interleukin-2 (IL-2), but did block the coupling of these events to DNA synthesis and proliferation.
Pharmacokinetics
Following oral and intravenous administration, mycophenolate mofetil undergoes rapid and complete metabolism to MPA, the active metabolite. Oral absorption of the drug is rapid and essentially complete. MPA is metabolized to form the phenolic glucuronide of MPA (MPAG) which is not pharmacologically active. The parent drug, mycophenolate mofetil, can be measured systemically during the intravenous infusion; however, shortly (about 5 minutes) after the infusion is stopped or after oral administration, MMF concentration is below the limit of quantitation (0.4 µg/mL).
Absorption
In 12 healthy volunteers, the mean absolute bioavailability of oral mycophenolate mofetil relative to intravenous mycophenolate mofetil (based on MPA AUC) was 94%. The area under the plasma-concentration time curve (AUC) for MPA appears to increase in a dose-proportional fashion in renal transplant patients receiving multiple doses of mycophenolate mofetil up to a daily dose of 3 g (see Table 1).
Food (27 g fat, 650 calories) had no effect on the extent of absorption (MPA AUC) of mycophenolate mofetil when administered at doses of 1.5 g bid to renal transplant patients. However, MPA Cmax was decreased by 40% in the presence of food (see DOSAGE AND ADMINISTRATION).
Distribution
The mean (±SD) apparent volume of distribution of MPA in 12 healthy volunteers is approximately 3.6 (±1.5) and 4.0 (±1.2) L/kg following intravenous and oral administration, respectively. MPA, at clinically relevant concentrations, is 97% bound to plasma albumin. MPAG is 82% bound to plasma albumin at MPAG concentration ranges that are normally seen in stable renal transplant patients; however, at higher MPAG concentrations (observed in patients with renal impairment or delayed renal graft function), the binding of MPA may be reduced as a result of competition between MPAG and MPA for protein binding. Mean blood to plasma ratio of radioactivity concentrations was approximately 0.6 indicating that MPA and MPAG do not extensively distribute into the cellular fractions of blood.
In vitro studies to evaluate the effect of other agents on the binding of MPA to human serum albumin (HSA) or plasma proteins showed that salicylate (at 25 mg/dL with HSA) and MPAG (at ≥460 µg/mL with plasma proteins) increased the free fraction of MPA. At concentrations that exceeded what is encountered clinically, cyclosporine, digoxin, naproxen, prednisone, propranolol, tacrolimus, theophylline, tolbutamide, and warfarin did not increase the free fraction of MPA. MPA at concentrations as high as 100 µg/mL had little effect on the binding of warfarin, digoxin or propranolol, but decreased the binding of theophylline from 53% to 45% and phenytoin from 90% to 87%.
Metabolism
Following oral and intravenous dosing, mycophenolate mofetil undergoes complete metabolism to MPA, the active metabolite. Metabolism to MPA occurs presystemically after oral dosing. MPA is metabolized principally by glucuronyl transferase to form the phenolic glucuronide of MPA (MPAG) which is not pharmacologically active. In vivo, MPAG is converted to MPA via enterohepatic recirculation. The following metabolites of the 2-hydroxyethyl-morpholino moiety are also recovered in the urine following oral administration of mycophenolate mofetil to healthy subjects: N-(2-carboxymethyl)-morpholine, N-(2-hydroxyethyl)-morpholine, and the N-oxide of N-(2-hydroxyethyl)-morpholine.
Secondary peaks in the plasma MPA concentration-time profile are usually observed 6 to 12 hours postdose. The coadministration of cholestyramine (4 g tid) resulted in approximately a 40% decrease in the MPA AUC (largely as a consequence of lower concentrations in the terminal portion of the profile). These observations suggest that enterohepatic recirculation contributes to MPA plasma concentrations.
Increased plasma concentrations of mycophenolate mofetil metabolites (MPA 50% increase and MPAG about a 3-fold to 6-fold increase) are observed in patients with renal insufficiency (see CLINICAL PHARMACOLOGY: Special Populations).
Excretion
Negligible amount of drug is excreted as MPA (<1% of dose) in the urine. Orally administered radiolabeled mycophenolate mofetil resulted in complete recovery of the administered dose, with 93% of the administered dose recovered in the urine and 6% recovered in feces. Most (about 87%) of the administered dose is excreted in the urine as MPAG. At clinically encountered concentrations, MPA and MPAG are usually not removed by hemodialysis. However, at high MPAG plasma concentrations (>100 µg/mL), small amounts of MPAG are removed. Bile acid sequestrants, such as cholestyramine, reduce MPA AUC by interfering with enterohepatic circulation of the drug (see OVERDOSAGE).
Mean (±SD) apparent half-life and plasma clearance of MPA are 17.9 (±6.5) hours and 193 (±48) mL/min following oral administration and 16.6 (±5.8) hours and 177 (±31) mL/min following intravenous administration, respectively.
Pharmacokinetics in Healthy Volunteers, Renal, Cardiac, and Hepatic Transplant Patients
Shown below are the mean (±SD) pharmacokinetic parameters for MPA following the administration of mycophenolate mofetil given as single doses to healthy volunteers and multiple doses to renal, cardiac, and hepatic transplant patients. In the early posttransplant period (<40 days posttransplant), renal, cardiac, and hepatic transplant patients had mean MPA AUCs approximately 20% to 41% lower and mean Cmax approximately 32% to 44% lower compared to the late transplant period (3 to 6 months posttransplant).
Mean MPA AUC values following administration of 1 g bid intravenous mycophenolate mofetil over 2 hours to renal transplant patients for 5 days were about 24% higher than those observed after oral administration of a similar dose in the immediate posttransplant phase. In hepatic transplant patients, administration of 1 g bid intravenous CellCept followed by 1.5 g bid oral CellCept resulted in mean MPA AUC values similar to those found in renal transplant patients administered 1 g CellCept bid.
Table 1 Pharmacokinetic Parameters for MPA [mean (±SD)] Following Administration of Mycophenolate Mofetil to Healthy Volunteers (Single Dose), Renal, Cardiac, and Hepatic Transplant Patients (Multiple Doses) - * AUC(0-12h) values quoted are extrapolated from data from samples collected over 4 hours.
Dose/Route Tmax
(h)Cmax
(µg/mL)Total AUC
(µg∙h/mL)Healthy Volunteers
(single dose)1 g/oral 0.80
(±0.36)
(n=129)24.5
(±9.5)
(n=129)63.9
(±16.2)
(n=117)Renal Transplant Patients (bid dosing) Time After Transplantation Dose/Route Tmax
(h)Cmax
(µg/mL)Interdosing Interval AUC(0-12h)
(µg∙h/mL)5 days 1 g/iv 1.58
(±0.46)
(n=31)12.0
(±3.82)
(n=31)40.8
(±11.4)
(n=31)6 days 1 g/oral 1.33
(±1.05)
(n=31)10.7
(±4.83)
(n=31)32.9
(±15.0)
(n=31)Early (<40 days) 1 g/oral 1.31
(±0.76)
(n=25)8.16
(±4.50)
(n=25)27.3
(±10.9)
(n=25)Early (<40 days) 1.5 g/oral 1.21
(±0.81)
(n=27)13.5
(±8.18)
(n=27)38.4
(±15.4)
(n=27)Late (>3 months) 1.5 g/oral 0.90
(±0.24)
(n=23)24.1
(±12.1)
(n=23)65.3
(±35.4)
(n=23)Cardiac Transplant Patients (bid dosing) Time After Transplantation Dose/Route Tmax
(h)Cmax
(µg/mL)Interdosing Interval AUC(0-12h)
(µg∙h/mL)Early
(Day before discharge)1.5 g/oral 1.8
(±1.3)
(n=11)11.5
(±6.8)
(n=11)43.3
(±20.8)
(n=9)Late (>6 months) 1.5 g/oral 1.1
(±0.7)
(n=52)20.0
(±9.4)
(n=52)54.1*
(±20.4)
(n=49)Hepatic Transplant Patients (bid dosing) Time After Transplantation Dose/Route Tmax
(h)Cmax
(µg/mL)Interdosing Interval AUC(0-12h)
(µg∙h/mL)4 to 9 days 1 g/iv 1.50
(±0.517)
(n=22)17.0
(±12.7)
(n=22)34.0
(±17.4)
(n=22)Early (5 to 8 days) 1.5 g/oral 1.15
(±0.432)
(n=20)13.1
(±6.76)
(n=20)29.2
(±11.9)
(n=20)Late (>6 months) 1.5 g/oral 1.54
(±0.51)
(n=6)19.3
(±11.7)
(n=6)49.3
(±14.8)
(n=6)Two 500 mg tablets have been shown to be bioequivalent to four 250 mg capsules. Five mL of the 200 mg/mL constituted oral suspension have been shown to be bioequivalent to four 250 mg capsules.
Special Populations
Shown below are the mean (±SD) pharmacokinetic parameters for MPA following the administration of oral mycophenolate mofetil given as single doses to non-transplant subjects with renal or hepatic impairment.
Table 2 Pharmacokinetic Parameters for MPA [mean (±SD)] Following Single Doses of Mycophenolate Mofetil Capsules in Chronic Renal and Hepatic Impairment Renal Impairment
(no. of patients)Dose Tmax
(h)Cmax
(µg/mL)AUC(0-96h)
(µg∙h/mL)Healthy Volunteers
GFR >80 mL/min/1.73 m2
(n=6)1 g 0.75
(±0.27)25.3
(±7.99)45.0
(±22.6)Mild Renal Impairment
GFR 50 to 80 mL/min/1.73 m2
(n=6)1 g 0.75
(±0.27)26.0
(±3.82)59.9
(±12.9)Moderate Renal Impairment
GFR 25 to 49 mL/min/1.73 m2
(n=6)1 g 0.75
(±0.27)19.0
(±13.2)52.9
(±25.5)Severe Renal Impairment
GFR <25 mL/min/1.73 m2
(n=7)1 g 1.00
(±0.41)16.3
(±10.8)78.6
(±46.4)Hepatic Impairment
(no. of patients)Dose Tmax
(h)Cmax
(µg/mL)AUC(0-48h)
(µg∙h/mL)Healthy Volunteers
(n=6)1 g 0.63
(±0.14)24.3
(±5.73)29.0
(±5.78)Alcoholic Cirrhosis
(n=18)1 g 0.85
(±0.58)22.4
(±10.1)29.8
(±10.7)Renal Insufficiency
In a single-dose study, MMF was administered as capsule or intravenous infusion over 40 minutes. Plasma MPA AUC observed after oral dosing to volunteers with severe chronic renal impairment [glomerular filtration rate (GFR) <25 mL/min/1.73 m2] was about 75% higher relative to that observed in healthy volunteers (GFR >80 mL/min/1.73 m2). In addition, the single-dose plasma MPAG AUC was 3-fold to 6-fold higher in volunteers with severe renal impairment than in volunteers with mild renal impairment or healthy volunteers, consistent with the known renal elimination of MPAG. No data are available on the safety of long-term exposure to this level of MPAG.
Plasma MPA AUC observed after single-dose (1 g) intravenous dosing to volunteers (n=4) with severe chronic renal impairment (GFR <25 mL/min/1.73 m2) was 62.4 µg∙h/mL (±19.3). Multiple dosing of mycophenolate mofetil in patients with severe chronic renal impairment has not been studied (see PRECAUTIONS: General and DOSAGE AND ADMINISTRATION).
In patients with delayed renal graft function posttransplant, mean MPA AUC(0-12h) was comparable to that seen in posttransplant patients without delayed renal graft function. There is a potential for a transient increase in the free fraction and concentration of plasma MPA in patients with delayed renal graft function. However, dose adjustment does not appear to be necessary in patients with delayed renal graft function. Mean plasma MPAG AUC(0-12h) was 2-fold to 3-fold higher than in posttransplant patients without delayed renal graft function (see PRECAUTIONS: General and DOSAGE AND ADMINISTRATION).
In 8 patients with primary graft non-function following renal transplantation, plasma concentrations of MPAG accumulated about 6-fold to 8-fold after multiple dosing for 28 days. Accumulation of MPA was about 1-fold to 2-fold.
The pharmacokinetics of mycophenolate mofetil are not altered by hemodialysis. Hemodialysis usually does not remove MPA or MPAG. At high concentrations of MPAG (>100 µg/mL), hemodialysis removes only small amounts of MPAG.
Hepatic Insufficiency
In a single-dose (1 g oral) study of 18 volunteers with alcoholic cirrhosis and 6 healthy volunteers, hepatic MPA glucuronidation processes appeared to be relatively unaffected by hepatic parenchymal disease when pharmacokinetic parameters of healthy volunteers and alcoholic cirrhosis patients within this study were compared. However, it should be noted that for unexplained reasons, the healthy volunteers in this study had about a 50% lower AUC as compared to healthy volunteers in other studies, thus making comparisons between volunteers with alcoholic cirrhosis and healthy volunteers difficult. Effects of hepatic disease on this process probably depend on the particular disease. Hepatic disease with other etiologies, such as primary biliary cirrhosis, may show a different effect. In a single-dose (1 g intravenous) study of 6 volunteers with severe hepatic impairment (aminopyrine breath test less than 0.2% of dose) due to alcoholic cirrhosis, MMF was rapidly converted to MPA. MPA AUC was 44.1 µg∙h/mL (±15.5).
Pediatrics
The pharmacokinetic parameters of MPA and MPAG have been evaluated in 55 pediatric patients (ranging from 1 year to 18 years of age) receiving CellCept oral suspension at a dose of 600 mg/m2 bid (up to a maximum of 1 g bid) after allogeneic renal transplantation. The pharmacokinetic data for MPA is provided in Table 3:
Table 3 Mean (±SD) Computed Pharmacokinetic Parameters for MPA by Age and Time After Allogeneic Renal Transplantation Age Group (n) Time Tmax
(h)Dose Adjusted*
Cmax
(µg/mL)Dose Adjusted*
AUC0-12
(µg∙h/mL)- * adjusted to a dose of 600 mg/m2
- † a subset of 1 to <6 yr
- ‡ n=20
- § n=16
Early (Day 7)
1 to <2 yr(6)† 3.03 (4.70) 10.3 (5.80) 22.5 (6.66)
1 to <6 yr (17) 1.63 (2.85) 13.2 (7.16) 27.4 (9.54) 6 to <12 yr (16) 0.940 (0.546) 13.1 (6.30) 33.2 (12.1) 12 to 18 yr (21) 1.16 (0.830) 11.7 (10.7) 26.3 (9.14)‡ Late (Month 3) 1 to <2 yr (4)† 0.725 (0.276) 23.8 (13.4) 47.4 (14.7) 1 to <6 yr (15) 0.989 (0.511) 22.7 (10.1) 49.7 (18.2) 6 to <12 yr (14) 1.21 (0.532) 27.8 (14.3) 61.9 (19.6) 12 to 18 yr (17) 0.978 (0.484) 17.9 (9.57) 53.6 (20.3)§ Late (Month 9) 1 to <2 yr (4)† 0.604 (0.208) 25.6 (4.25) 55.8 (11.6) 1 to <6 yr (12) 0.869 (0.479) 30.4 (9.16) 61.0 (10.7) 6 to <12 yr (11) 1.12 (0.462) 29.2 (12.6) 66.8 (21.2) 12 to 18 yr (14) 1.09 (0.518) 18.1 (7.29) 56.7 (14.0) The CellCept oral suspension dose of 600 mg/m2 bid (up to a maximum of 1 g bid) achieved mean MPA AUC values in pediatric patients similar to those seen in adult renal transplant patients receiving CellCept capsules at a dose of 1 g bid in the early posttransplant period. There was wide variability in the data. As observed in adults, early posttransplant MPA AUC values were approximately 45% to 53% lower than those observed in the later posttransplant period (>3 months). MPA AUC values were similar in the early and late posttransplant period across the 1 year to 18 year age range.
Gender
Data obtained from several studies were pooled to look at any gender-related differences in the pharmacokinetics of MPA (data were adjusted to 1 g oral dose). Mean (±SD) MPA AUC(0-12h) for males (n=79) was 32.0 (±14.5) and for females (n=41) was 36.5 (±18.8) µg∙h/mL while mean (±SD) MPA Cmax was 9.96 (±6.19) in the males and 10.6 (±5.64) µg/mL in the females. These differences are not of clinical significance.
-
CLINICAL STUDIES
Adults
The safety and efficacy of CellCept in combination with corticosteroids and cyclosporine for the prevention of organ rejection were assessed in randomized, double-blind, multicenter trials in renal (3 trials), in cardiac (1 trial), and in hepatic (1 trial) adult transplant patients.
Renal Transplant
Adults
The three renal studies compared two dose levels of oral CellCept (1 g bid and 1.5 g bid) with azathioprine (2 studies) or placebo (1 study) when administered in combination with cyclosporine (Sandimmune®) and corticosteroids to prevent acute rejection episodes. One study also included antithymocyte globulin (ATGAM®) induction therapy. These studies are described by geographic location of the investigational sites. One study was conducted in the USA at 14 sites, one study was conducted in Europe at 20 sites, and one study was conducted in Europe, Canada, and Australia at a total of 21 sites.
The primary efficacy endpoint was the proportion of patients in each treatment group who experienced treatment failure within the first 6 months after transplantation (defined as biopsy-proven acute rejection on treatment or the occurrence of death, graft loss or early termination from the study for any reason without prior biopsy-proven rejection). CellCept, when administered with antithymocyte globulin (ATGAM®) induction (one study) and with cyclosporine and corticosteroids (all three studies), was compared to the following three therapeutic regimens: (1) antithymocyte globulin (ATGAM®) induction/azathioprine/cyclosporine/corticosteroids, (2) azathioprine/cyclosporine/corticosteroids, and (3) cyclosporine/corticosteroids.
CellCept, in combination with corticosteroids and cyclosporine reduced (statistically significant at 0.05 level) the incidence of treatment failure within the first 6 months following transplantation. Table 4 and Table 5 summarize the results of these studies. These tables show (1) the proportion of patients experiencing treatment failure, (2) the proportion of patients who experienced biopsy-proven acute rejection on treatment, and (3) early termination, for any reason other than graft loss or death, without a prior biopsy-proven acute rejection episode. Patients who prematurely discontinued treatment were followed for the occurrence of death or graft loss, and the cumulative incidence of graft loss and patient death are summarized separately. Patients who prematurely discontinued treatment were not followed for the occurrence of acute rejection after termination. More patients receiving CellCept discontinued without prior biopsy-proven rejection, death or graft loss than discontinued in the control groups, with the highest rate in the CellCept 3 g/day group. Therefore, the acute rejection rates may be underestimates, particularly in the CellCept 3 g/day group.
Table 4 Renal Transplant Studies Incidence of Treatment Failure (Biopsy-proven Rejection or Early Termination for Any Reason) - * Antithymocyte globulin induction/MMF or azathioprine/cyclosporine/corticosteroids.
- † Does not include death and graft loss as reason for early termination.
- ‡ MMF or azathioprine/cyclosporine/corticosteroids.
- § MMF or placebo/cyclosporine/corticosteroids.
USA Study*
(N=499 patients)CellCept
2 g/day
(n=167 patients)CellCept
3 g/day
(n=166 patients)Azathioprine
1 to 2 mg/kg/day
(n=166 patients)All treatment failures 31.1% 31.3% 47.6% Early termination without prior acute rejection† 9.6% 12.7% 6.0% Biopsy-proven rejection episode on treatment 19.8% 17.5% 38.0% Europe/Canada/
Australia Study‡
(N=503 patients)CellCept
2 g/day
(n=173 patients)CellCept
3 g/day
(n=164 patients)Azathioprine
100 to 150 mg/day
(n=166 patients)All treatment failures 38.2% 34.8% 50.0% Early termination without prior acute rejection† 13.9% 15.2% 10.2% Biopsy-proven rejection episode on treatment 19.7% 15.9% 35.5% Europe Study§
(N=491 patients)CellCept
2 g/day
(n=165 patients)CellCept
3 g/day
(n=160 patients)Placebo
(n=166 patients)All treatment failures 30.3% 38.8% 56.0% Early termination without prior acute rejection† 11.5% 22.5% 7.2% Biopsy-proven rejection episode on treatment 17.0% 13.8% 46.4% The cumulative incidence of 12-month graft loss or patient death is presented below. No advantage of CellCept with respect to graft loss or patient death was established. Numerically, patients receiving CellCept 2 g/day and 3 g/day experienced a better outcome than controls in all three studies; patients receiving CellCept 2 g/day experienced a better outcome than CellCept 3 g/day in two of the three studies. Patients in all treatment groups who terminated treatment early were found to have a poor outcome with respect to graft loss or patient death at 1 year.
Table 5 Renal Transplant Studies Cumulative Incidence of Combined Graft Loss or Patient Death at 12 Months Study CellCept
2 g/dayCellCept
3 g/dayControl
(Azathioprine or Placebo)USA 8.5% 11.5% 12.2% Europe/Canada/Australia 11.7% 11.0% 13.6% Europe 8.5% 10.0% 11.5% Pediatrics
One open-label, safety and pharmacokinetic study of CellCept oral suspension 600 mg/m2 bid (up to 1 g bid) in combination with cyclosporine and corticosteroids was performed at centers in the US (9), Europe (5) and Australia (1) in 100 pediatric patients (3 months to 18 years of age) for the prevention of renal allograft rejection. CellCept was well tolerated in pediatric patients (see ADVERSE REACTIONS), and the pharmacokinetics profile was similar to that seen in adult patients dosed with 1 g bid CellCept capsules (see CLINICAL PHARMACOLOGY: Pharmacokinetics). The rate of biopsy-proven rejection was similar across the age groups (3 months to <6 years, 6 years to <12 years, 12 years to 18 years). The overall biopsy-proven rejection rate at 6 months was comparable to adults. The combined incidence of graft loss (5%) and patient death (2%) at 12 months posttransplant was similar to that observed in adult renal transplant patients.
Cardiac Transplant
A double-blind, randomized, comparative, parallel-group, multicenter study in primary cardiac transplant recipients was performed at 20 centers in the United States, 1 in Canada, 5 in Europe and 2 in Australia. The total number of patients enrolled was 650; 72 never received study drug and 578 received study drug. Patients received CellCept 1.5 g bid (n=289) or azathioprine 1.5 to 3 mg/kg/day (n=289), in combination with cyclosporine (Sandimmune® or Neoral®) and corticosteroids as maintenance immunosuppressive therapy. The two primary efficacy endpoints were: (1) the proportion of patients who, after transplantation, had at least one endomyocardial biopsy-proven rejection with hemodynamic compromise, or were retransplanted or died, within the first 6 months, and (2) the proportion of patients who died or were retransplanted during the first 12 months following transplantation. Patients who prematurely discontinued treatment were followed for the occurrence of allograft rejection for up to 6 months and for the occurrence of death for 1 year.
(1) Rejection: No difference was established between CellCept and azathioprine (AZA) with respect to biopsy-proven rejection with hemodynamic compromise.
(2) Survival: CellCept was shown to be at least as effective as AZA in preventing death or retransplantation at 1 year (see Table 6).
Table 6 Rejection at 6 Months/Death or Retransplantation at 1 Year All Patients Treated Patients AZA
N = 323CellCept
N = 327AZA
N = 289CellCept
N = 289- * Hemodynamic compromise occurred if any of the following criteria were met: pulmonary capillary wedge pressure ≥20 mm or a 25% increase; cardiac index <2.0 L/min/m2 or a 25% decrease; ejection fraction ≤30%; pulmonary artery oxygen saturation ≤60% or a 25% decrease; presence of new S3 gallop; fractional shortening was ≤20% or a 25% decrease; inotropic support required to manage the clinical condition.
Biopsy-proven rejection with hemodynamic compromise at 6 months* 121 (38%) 120 (37%) 100 (35%) 92 (32%) Death or retransplantation at 1 year 49 (15.2%) 42 (12.8%) 33 (11.4%) 18 (6.2%) Hepatic Transplant
A double-blind, randomized, comparative, parallel-group, multicenter study in primary hepatic transplant recipients was performed at 16 centers in the United States, 2 in Canada, 4 in Europe and 1 in Australia. The total number of patients enrolled was 565. Per protocol, patients received CellCept 1 g bid intravenously for up to 14 days followed by CellCept 1.5 g bid orally or azathioprine 1 to 2 mg/kg/day intravenously followed by azathioprine 1 to 2 mg/kg/day orally, in combination with cyclosporine (Neoral®) and corticosteroids as maintenance immunosuppressive therapy. The actual median oral dose of azathioprine on study was 1.5 mg/kg/day (range of 0.3 to 3.8 mg/kg/day) initially and 1.26 mg/kg/day (range of 0.3 to 3.8 mg/kg/day) at 12 months. The two primary endpoints were: (1) the proportion of patients who experienced, in the first 6 months posttransplantation, one or more episodes of biopsy-proven and treated rejection or death or retransplantation, and (2) the proportion of patients who experienced graft loss (death or retransplantation) during the first 12 months posttransplantation. Patients who prematurely discontinued treatment were followed for the occurrence of allograft rejection and for the occurrence of graft loss (death or retransplantation) for 1 year.
Results
In combination with corticosteroids and cyclosporine, CellCept obtained a lower rate of acute rejection at 6 months and a similar rate of death or retransplantation at 1 year compared to azathioprine.
Table 7 Rejection at 6 Months/Death or Retransplantation at 1 Year AZA
N = 287CellCept
N = 278Biopsy-proven, treated rejection at 6 months (includes death or retransplantation) 137 (47.7%) 107 (38.5%) Death or retransplantation at 1 year 42 (14.6%) 41 (14.7%) -
INDICATIONS AND USAGE
Renal, Cardiac, and Hepatic Transplant
CellCept is indicated for the prophylaxis of organ rejection in patients receiving allogeneic renal, cardiac or hepatic transplants. CellCept should be used concomitantly with cyclosporine and corticosteroids.
CellCept Intravenous is an alternative dosage form to CellCept capsules, tablets and oral suspension. CellCept Intravenous should be administered within 24 hours following transplantation. CellCept Intravenous can be administered for up to 14 days; patients should be switched to oral CellCept as soon as they can tolerate oral medication.
-
CONTRAINDICATIONS
Allergic reactions to CellCept have been observed; therefore, CellCept is contraindicated in patients with a hypersensitivity to mycophenolate mofetil, mycophenolic acid or any component of the drug product. CellCept Intravenous is contraindicated in patients who are allergic to Polysorbate 80 (TWEEN).
-
WARNINGS
(see boxed WARNING)
Lymphoma and Malignancy
Patients receiving immunosuppressive regimens involving combinations of drugs, including CellCept, as part of an immunosuppressive regimen are at increased risk of developing lymphomas and other malignancies, particularly of the skin (see ADVERSE REACTIONS). The risk appears to be related to the intensity and duration of immunosuppression rather than to the use of any specific agent.
As usual for patients with increased risk for skin cancer, exposure to sunlight and UV light should be limited by wearing protective clothing and using a sunscreen with a high protection factor.
Lymphoproliferative disease or lymphoma developed in 0.4% to 1% of patients receiving CellCept (2 g or 3 g) with other immunosuppressive agents in controlled clinical trials of renal, cardiac, and hepatic transplant patients (see ADVERSE REACTIONS).
In pediatric patients, no other malignancies besides lymphoproliferative disorder (2/148 patients) have been observed (see ADVERSE REACTIONS).
Combination with Other Immunosuppressive Agents
CellCept has been administered in combination with the following agents in clinical trials: antithymocyte globulin (ATGAM®), OKT3 (Orthoclone OKT® 3), cyclosporine (Sandimmune®, Neoral®) and corticosteroids. The efficacy and safety of the use of CellCept in combination with other immunosuppressive agents have not been determined.
Infections
Oversuppression of the immune system can also increase susceptibility to infection, including opportunistic infections, fatal infections, and sepsis. In patients receiving CellCept (2 g or 3 g) in controlled studies for prevention of renal, cardiac or hepatic rejection, fatal infection/sepsis occurred in approximately 2% of renal and cardiac patients and in 5% of hepatic patients (see ADVERSE REACTIONS).
Latent Viral Infections
Immunosuppressed patients are at increased risk for opportunistic infections, including activation of latent viral infections. These include cases of progressive multifocal leukoencephalopathy (PML) and BK virus-associated nephropathy (BKVAN) which have been observed in patients receiving immunosuppressants, including CellCept.
Cases of progressive multifocal leukoencephalopathy (PML), sometimes fatal, have been reported in patients treated with CellCept. Hemiparesis, apathy, confusion, cognitive deficiencies and ataxia were the most frequent clinical features observed. The reported cases generally had risk factors for PML, including treatment with immunosuppressant therapies and impairment of immune function. In immunosuppressed patients, physicians should consider PML in the differential diagnosis in patients reporting neurological symptoms and consultation with a neurologist should be considered as clinically indicated. Consideration should be given to reducing the amount of immunosuppression in patients who develop PML. In transplant patients, physicians should also consider the risk that reduced immunosuppression represents to the graft.
BKVAN is associated with serious outcomes, including deteriorating renal function and renal graft loss (see ADVERSE REACTIONS: Postmarketing Experience). Patient monitoring may help detect patients at risk for BK virus-associated nephropathy. Reduction in immunosuppression should be considered for patients who develop evidence of BK virus-associated nephropathy.
Pregnancy
Teratogenic Effects
Pregnancy Category D
Mycophenolate mofetil (MMF) can cause fetal harm when administered to a pregnant woman. Use of MMF during pregnancy is associated with an increased risk of first trimester pregnancy loss and an increased risk of congenital malformations, especially external ear and other facial abnormalities including cleft lip and palate, and anomalies of the distal limbs, heart, esophagus, and kidney. In the National Transplantation Pregnancy Registry (NTPR), there were data on 33 MMF-exposed pregnancies in 24 transplant patients; there were 15 spontaneous abortions (45%) and 18 live-born infants. Four of these 18 infants had structural malformations (22%). In postmarketing data (collected 1995-2007) on 77 women exposed to systemic MMF during pregnancy, 25 had spontaneous abortions and 14 had a malformed infant or fetus. Six of 14 malformed offspring had ear abnormalities. Because these postmarketing data are reported voluntarily, it is not always possible to reliably estimate the frequency of particular adverse outcomes. These malformations seen in offspring were similar to findings in animal reproductive toxicology studies. For comparison, the background rate for congenital anomalies in the United States is about 3%, and NTPR data show a rate of 4-5% among babies born to organ transplant patients using other immunosuppressive drugs.
In animal reproductive toxicology studies, there were increased rates of fetal resorptions and malformations in the absence of maternal toxicity. Female rats and rabbits received mycophenolate mofetil (MMF) doses equivalent to 0.02 to 0.9 times the recommended human dose for renal and cardiac transplant patients, based on body surface area conversions. In rat offspring, malformations included anophthalmia, agnathia, and hydrocephaly. In rabbit offspring, malformations included ectopia cordis, ectopic kidneys, diaphragmatic hernia, and umbilical hernia.
If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to the fetus. In certain situations, the patient and her healthcare practitioner may decide that the maternal benefits outweigh the risks to the fetus. Women using CellCept at any time during pregnancy should be encouraged to enroll in the National Transplantation Pregnancy Registry.
Pregnancy Exposure Prevention
Women of childbearing potential should have a negative serum or urine pregnancy test with a sensitivity of at least 25 mIU/mL within 1 week prior to beginning therapy. CellCept therapy should not be initiated until a negative pregnancy test report is obtained.
Women of childbearing potential (including pubertal girls and peri-menopausal women) taking CellCept must receive contraceptive counseling and use effective contraception. The patient should begin using her two chosen methods of contraception 4 weeks prior to starting CellCept therapy, unless abstinence is the chosen method. She should continue contraceptive use during therapy and for 6 weeks after stopping CellCept. Patients should be aware that CellCept reduces blood levels of the hormones in the oral contraceptive pill and could theoretically reduce its effectiveness (see PRECAUTIONS: Information for Patients and PRECAUTIONS: Drug Interactions: Oral Contraceptives).
Neutropenia
Severe neutropenia [absolute neutrophil count (ANC) <0.5 × 103/µL] developed in up to 2.0% of renal, up to 2.8% of cardiac, and up to 3.6% of hepatic transplant patients receiving CellCept 3 g daily (see ADVERSE REACTIONS). Patients receiving CellCept should be monitored for neutropenia (see PRECAUTIONS: Laboratory Tests). The development of neutropenia may be related to CellCept itself, concomitant medications, viral infections, or some combination of these causes. If neutropenia develops (ANC <1.3 × 103/µL), dosing with CellCept should be interrupted or the dose reduced, appropriate diagnostic tests performed, and the patient managed appropriately (see DOSAGE AND ADMINISTRATION). Neutropenia has been observed most frequently in the period from 31 to 180 days posttransplant in patients treated for prevention of renal, cardiac, and hepatic rejection.
Patients receiving CellCept should be instructed to report immediately any evidence of infection, unexpected bruising, bleeding or any other manifestation of bone marrow depression.
Pure Red Cell Aplasia (PRCA)
Cases of pure red cell aplasia (PRCA) have been reported in patients treated with CellCept in combination with other immunosuppressive agents. The mechanism for mycophenolate mofetil induced PRCA is unknown; the relative contribution of other immunosuppressants and their combinations in an immunosuppression regimen are also unknown. In some cases, PRCA was found to be reversible with dose reduction or cessation of CellCept therapy. In transplant patients, however, reduced immunosuppression may place the graft at risk.
CAUTION: CELLCEPT INTRAVENOUS SOLUTION SHOULD NEVER BE ADMINISTERED BY RAPID OR BOLUS INTRAVENOUS INJECTION.
-
PRECAUTIONS
General
Gastrointestinal bleeding (requiring hospitalization) has been observed in approximately 3% of renal, in 1.7% of cardiac, and in 5.4% of hepatic transplant patients treated with CellCept 3 g daily. In pediatric renal transplant patients, 5/148 cases of gastrointestinal bleeding (requiring hospitalization) were observed.
Gastrointestinal perforations have rarely been observed. Most patients receiving CellCept were also receiving other drugs known to be associated with these complications. Patients with active peptic ulcer disease were excluded from enrollment in studies with mycophenolate mofetil. Because CellCept has been associated with an increased incidence of digestive system adverse events, including infrequent cases of gastrointestinal tract ulceration, hemorrhage, and perforation, CellCept should be administered with caution in patients with active serious digestive system disease.
Subjects with severe chronic renal impairment (GFR <25 mL/min/1.73 m2) who have received single doses of CellCept showed higher plasma MPA and MPAG AUCs relative to subjects with lesser degrees of renal impairment or normal healthy volunteers. No data are available on the safety of long-term exposure to these levels of MPAG. Doses of CellCept greater than 1 g administered twice a day to renal transplant patients should be avoided and they should be carefully observed (see CLINICAL PHARMACOLOGY: Pharmacokinetics and DOSAGE AND ADMINISTRATION).
No data are available for cardiac or hepatic transplant patients with severe chronic renal impairment. CellCept may be used for cardiac or hepatic transplant patients with severe chronic renal impairment if the potential benefits outweigh the potential risks.
In patients with delayed renal graft function posttransplant, mean MPA AUC(0-12h) was comparable, but MPAG AUC(0-12h) was 2-fold to 3-fold higher, compared to that seen in posttransplant patients without delayed renal graft function. In the three controlled studies of prevention of renal rejection, there were 298 of 1483 patients (20%) with delayed graft function. Although patients with delayed graft function have a higher incidence of certain adverse events (anemia, thrombocytopenia, hyperkalemia) than patients without delayed graft function, these events were not more frequent in patients receiving CellCept than azathioprine or placebo. No dose adjustment is recommended for these patients; however, they should be carefully observed (see CLINICAL PHARMACOLOGY: Pharmacokinetics and DOSAGE AND ADMINISTRATION).
In cardiac transplant patients, the overall incidence of opportunistic infections was approximately 10% higher in patients treated with CellCept than in those receiving azathioprine therapy, but this difference was not associated with excess mortality due to infection/sepsis among patients treated with CellCept (see ADVERSE REACTIONS).
There were more herpes virus (H. simplex, H. zoster, and cytomegalovirus) infections in cardiac transplant patients treated with CellCept compared to those treated with azathioprine (see ADVERSE REACTIONS).
It is recommended that CellCept not be administered concomitantly with azathioprine because both have the potential to cause bone marrow suppression and such concomitant administration has not been studied clinically.
In view of the significant reduction in the AUC of MPA by cholestyramine, caution should be used in the concomitant administration of CellCept with drugs that interfere with enterohepatic recirculation because of the potential to reduce the efficacy of CellCept (see PRECAUTIONS: Drug Interactions).
On theoretical grounds, because CellCept is an IMPDH (inosine monophosphate dehydrogenase) inhibitor, it should be avoided in patients with rare hereditary deficiency of hypoxanthine-guanine phosphoribosyl-transferase (HGPRT) such as Lesch-Nyhan and Kelley-Seegmiller syndrome.
During treatment with CellCept, the use of live attenuated vaccines should be avoided and patients should be advised that vaccinations may be less effective (see PRECAUTIONS: Drug Interactions: Live Vaccines).
Phenylketonurics
CellCept Oral Suspension contains aspartame, a source of phenylalanine (0.56 mg phenylalanine/mL suspension). Therefore, care should be taken if CellCept Oral Suspension is administered to patients with phenylketonuria.
Information for Patients
- Give patients complete dosage instructions and inform them about the increased risk of lymphoproliferative disease and certain other malignancies.
- Inform patients that they need repeated appropriate laboratory tests while they are taking CellCept.
- Inform women of childbearing potential that use of CellCept in pregnancy is associated with an increased risk of first trimester pregnancy loss and an increased risk of birth defects, and that they must use effective contraception.
- Discuss pregnancy plans with female patients of childbearing potential.
- ♦Any female of childbearing potential must use highly effective (two methods) contraception 4 weeks prior to starting CellCept therapy and continue contraception until 6 weeks after stopping CellCept treatment, unless abstinence is the chosen method (see WARNINGS: Pregnancy).
- ♦A patient who is planning a pregnancy should not use CellCept unless she cannot be successfully treated with other immunosuppressant drugs.
Laboratory Tests
Complete blood counts should be performed weekly during the first month, twice monthly for the second and third months of treatment, then monthly through the first year (see WARNINGS, ADVERSE REACTIONS and DOSAGE AND ADMINISTRATION).
Drug Interactions
Drug interaction studies with mycophenolate mofetil have been conducted with acyclovir, antacids, cholestyramine, cyclosporine, ganciclovir, oral contraceptives, sevelamer, trimethoprim/sulfamethoxazole, norfloxacin, and metronidazole. Drug interaction studies have not been conducted with other drugs that may be commonly administered to renal, cardiac or hepatic transplant patients. CellCept has not been administered concomitantly with azathioprine.
Acyclovir
Coadministration of mycophenolate mofetil (1 g) and acyclovir (800 mg) to 12 healthy volunteers resulted in no significant change in MPA AUC and Cmax. However, MPAG and acyclovir plasma AUCs were increased 10.6% and 21.9%, respectively. Because MPAG plasma concentrations are increased in the presence of renal impairment, as are acyclovir concentrations, the potential exists for mycophenolate and acyclovir or its prodrug (eg, valacyclovir) to compete for tubular secretion, further increasing the concentrations of both drugs.
Antacids With Magnesium and Aluminum Hydroxides
Absorption of a single dose of mycophenolate mofetil (2 g) was decreased when administered to ten rheumatoid arthritis patients also taking Maalox® TC (10 mL qid). The Cmax and AUC(0-24h) for MPA were 33% and 17% lower, respectively, than when mycophenolate mofetil was administered alone under fasting conditions. CellCept may be administered to patients who are also taking antacids containing magnesium and aluminum hydroxides; however, it is recommended that CellCept and the antacid not be administered simultaneously.
Cholestyramine
Following single-dose administration of 1.5 g mycophenolate mofetil to 12 healthy volunteers pretreated with 4 g tid of cholestyramine for 4 days, MPA AUC decreased approximately 40%. This decrease is consistent with interruption of enterohepatic recirculation which may be due to binding of recirculating MPAG with cholestyramine in the intestine. Some degree of enterohepatic recirculation is also anticipated following intravenous administration of CellCept. Therefore, CellCept is not recommended to be given with cholestyramine or other agents that may interfere with enterohepatic recirculation.
Cyclosporine
Cyclosporine (Sandimmune®) pharmacokinetics (at doses of 275 to 415 mg/day) were unaffected by single and multiple doses of 1.5 g bid of mycophenolate mofetil in 10 stable renal transplant patients. The mean (±SD) AUC(0-12h) and Cmax of cyclosporine after 14 days of multiple doses of mycophenolate mofetil were 3290 (±822) ng∙h/mL and 753 (±161) ng/mL, respectively, compared to 3245 (±1088) ng∙h/mL and 700 (±246) ng/mL, respectively, 1 week before administration of mycophenolate mofetil.
In renal transplant patients, mean MPA exposure (AUC0-12h) was approximately 30-50% greater when mycophenolate mofetil is administered without cyclosporine compared with when mycophenolate mofetil is coadministered with cyclosporine. This interaction is due to cyclosporine inhibition of multidrug-resistance-associated protein 2 (MRP-2) transporter in the biliary tract, thereby preventing the excretion of MPAG into the bile that would lead to enterohepatic recirculation of MPA. This information should be taken into consideration when MMF is used without cyclosporine.
Ganciclovir
Following single-dose administration to 12 stable renal transplant patients, no pharmacokinetic interaction was observed between mycophenolate mofetil (1.5 g) and intravenous ganciclovir (5 mg/kg). Mean (±SD) ganciclovir AUC and Cmax (n=10) were 54.3 (±19.0) µg∙h/mL and 11.5 (±1.8) µg/mL, respectively, after coadministration of the two drugs, compared to 51.0 (±17.0) µg∙h/mL and 10.6 (±2.0) µg/mL, respectively, after administration of intravenous ganciclovir alone. The mean (±SD) AUC and Cmax of MPA (n=12) after coadministration were 80.9 (±21.6) µg∙h/mL and 27.8 (±13.9) µg/mL, respectively, compared to values of 80.3 (±16.4) µg∙h/mL and 30.9 (±11.2) µg/mL, respectively, after administration of mycophenolate mofetil alone. Because MPAG plasma concentrations are increased in the presence of renal impairment, as are ganciclovir concentrations, the two drugs will compete for tubular secretion and thus further increases in concentrations of both drugs may occur. In patients with renal impairment in which MMF and ganciclovir or its prodrug (eg, valganciclovir) are coadministered, patients should be monitored carefully.
Oral Contraceptives
A study of coadministration of CellCept (1 g bid) and combined oral contraceptives containing ethinylestradiol (0.02 mg to 0.04 mg) and levonorgestrel (0.05 mg to 0.20 mg), desogestrel (0.15 mg) or gestodene (0.05 mg to 0.10 mg) was conducted in 18 women with psoriasis over 3 consecutive menstrual cycles. Mean AUC(0-24h) was similar for ethinylestradiol and 3-keto desogestrel; however, mean levonorgestrel AUC(0-24h) significantly decreased by about 15%. There was large inter-patient variability (%CV in the range of 60% to 70%) in the data, especially for ethinylestradiol. Mean serum levels of LH, FSH and progesterone were not significantly affected. CellCept may not have any influence on the ovulation-suppressing action of the studied oral contraceptives. However, it is recommended that oral contraceptives are coadministered with CellCept with caution and additional birth control methods be considered (see WARNINGS: Pregnancy).
Sevelamer
Concomitant administration of sevelamer and mycophenolate mofetil in adult and pediatric patients decreased the mean MPA Cmax and AUC0-12h by 36% and 26% respectively. This data suggest that sevelamer and other calcium free phosphate binders should not be administered simultaneously with CellCept. Alternatively, it is recommended that sevelamer and other calcium free phosphate binders preferentially could be given 2 hours after CellCept intake to minimize the impact on the absorption of MPA.
Trimethoprim/sulfamethoxazole
Following single-dose administration of mycophenolate mofetil (1.5 g) to 12 healthy male volunteers on day 8 of a 10 day course of trimethoprim 160 mg/sulfamethoxazole 800 mg administered bid, no effect on the bioavailability of MPA was observed. The mean (±SD) AUC and Cmax of MPA after concomitant administration were 75.2 (±19.8) µg∙h/mL and 34.0 (±6.6) µg/mL, respectively, compared to 79.2 (±27.9) µg∙h/mL and 34.2 (±10.7) µg/mL, respectively, after administration of mycophenolate mofetil alone.
Norfloxacin and Metronidazole
Following single-dose administration of mycophenolate mofetil (1 g) to 11 healthy volunteers on day 4 of a 5 day course of a combination of norfloxacin and metronidazole, the mean MPA AUC0-48h was significantly reduced by 33% compared to the administration of mycophenolate mofetil alone (p<0.05). Therefore, CellCept is not recommended to be given with the combination of norfloxacin and metronidazole. There was no significant effect on mean MPA AUC0-48h when mycophenolate mofetil was concomitantly administered with norfloxacin or metronidazole separately. The mean (±SD) MPA AUC0-48h after coadministration of mycophenolate mofetil with norfloxacin or metronidazole separately was 48.3 (±24) µg∙h/mL and 42.7 (±23) µg∙h/mL, respectively, compared with 56.2 (±24) µg∙h/mL after administration of mycophenolate mofetil alone.
Ciprofloxacin and Amoxicillin plus Clavulanic Acid
A total of 64 CellCept-treated renal transplant recipients received either oral ciprofloxacin 500 mg bid or amoxicillin plus clavulanic acid 375 mg tid for 7 or at least 14 days. Approximately 50% reductions in median trough MPA concentrations (pre-dose) from baseline (CellCept alone) were observed in 3 days following commencement of oral ciprofloxacin or amoxicillin plus clavulanic acid. These reductions in trough MPA concentrations tended to diminish within 14 days of antibiotic therapy and ceased within 3 days after discontinuation of antibiotics. The postulated mechanism for this interaction is an antibiotic-induced reduction in glucuronidase-possessing enteric organisms leading to a decrease in enterohepatic recirculation of MPA. The change in trough level may not accurately represent changes in overall MPA exposure; therefore, clinical relevance of these observations is unclear.
Rifampin
In a single heart-lung transplant patient, after correction for dose, a 67% decrease in MPA exposure (AUC0-12h) has been observed with concomitant administration of mycophenolate mofetil and rifampin. Therefore, CellCept is not recommended to be given with rifampin concomitantly unless the benefit outweighs the risk.
Other Interactions
The measured value for renal clearance of MPAG indicates removal occurs by renal tubular secretion as well as glomerular filtration. Consistent with this, coadministration of probenecid, a known inhibitor of tubular secretion, with mycophenolate mofetil in monkeys results in a 3-fold increase in plasma MPAG AUC and a 2-fold increase in plasma MPA AUC. Thus, other drugs known to undergo renal tubular secretion may compete with MPAG and thereby raise plasma concentrations of MPAG or the other drug undergoing tubular secretion.
Drugs that alter the gastrointestinal flora may interact with mycophenolate mofetil by disrupting enterohepatic recirculation. Interference of MPAG hydrolysis may lead to less MPA available for absorption.
Live Vaccines
During treatment with CellCept, the use of live attenuated vaccines should be avoided and patients should be advised that vaccinations may be less effective (see PRECAUTIONS: General). Influenza vaccination may be of value. Prescribers should refer to national guidelines for influenza vaccination.
Carcinogenesis, Mutagenesis, Impairment of Fertility
In a 104-week oral carcinogenicity study in mice, mycophenolate mofetil in daily doses up to 180 mg/kg was not tumorigenic. The highest dose tested was 0.5 times the recommended clinical dose (2 g/day) in renal transplant patients and 0.3 times the recommended clinical dose (3 g/day) in cardiac transplant patients when corrected for differences in body surface area (BSA). In a 104-week oral carcinogenicity study in rats, mycophenolate mofetil in daily doses up to 15 mg/kg was not tumorigenic. The highest dose was 0.08 times the recommended clinical dose in renal transplant patients and 0.05 times the recommended clinical dose in cardiac transplant patients when corrected for BSA. While these animal doses were lower than those given to patients, they were maximal in those species and were considered adequate to evaluate the potential for human risk (see WARNINGS).
The genotoxic potential of mycophenolate mofetil was determined in five assays. Mycophenolate mofetil was genotoxic in the mouse lymphoma/thymidine kinase assay and the in vivo mouse micronucleus assay. Mycophenolate mofetil was not genotoxic in the bacterial mutation assay, the yeast mitotic gene conversion assay or the Chinese hamster ovary cell chromosomal aberration assay.
Mycophenolate mofetil had no effect on fertility of male rats at oral doses up to 20 mg/kg/day. This dose represents 0.1 times the recommended clinical dose in renal transplant patients and 0.07 times the recommended clinical dose in cardiac transplant patients when corrected for BSA. In a female fertility and reproduction study conducted in rats, oral doses of 4.5 mg/kg/day caused malformations (principally of the head and eyes) in the first generation offspring in the absence of maternal toxicity. This dose was 0.02 times the recommended clinical dose in renal transplant patients and 0.01 times the recommended clinical dose in cardiac transplant patients when corrected for BSA. No effects on fertility or reproductive parameters were evident in the dams or in the subsequent generation.
Nursing Mothers
Studies in rats treated with mycophenolate mofetil have shown mycophenolic acid to be excreted in milk. It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, and because of the potential for serious adverse reactions in nursing infants from mycophenolate mofetil, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
Based on pharmacokinetic and safety data in pediatric patients after renal transplantation, the recommended dose of CellCept oral suspension is 600 mg/m2 bid (up to a maximum of 1 g bid). Also see CLINICAL PHARMACOLOGY, CLINICAL STUDIES, ADVERSE REACTIONS, and DOSAGE AND ADMINISTRATION.
Safety and effectiveness in pediatric patients receiving allogeneic cardiac or hepatic transplants have not been established.
Geriatric Use
Clinical studies of CellCept did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general dose selection for an elderly patient should be cautious, reflecting the greater frequency of decreased hepatic, renal or cardiac function and of concomitant or other drug therapy. Elderly patients may be at an increased risk of adverse reactions compared with younger individuals (see ADVERSE REACTIONS).
-
ADVERSE REACTIONS
The principal adverse reactions associated with the administration of CellCept include diarrhea, leukopenia, sepsis, vomiting, and there is evidence of a higher frequency of certain types of infections eg, opportunistic infection (see WARNINGS: Infections and WARNINGS: Latent Viral Infections). The adverse event profile associated with the administration of CellCept Intravenous has been shown to be similar to that observed after administration of oral dosage forms of CellCept.
CellCept Oral
The incidence of adverse events for CellCept was determined in randomized, comparative, double-blind trials in prevention of rejection in renal (2 active, 1 placebo-controlled trials), cardiac (1 active-controlled trial), and hepatic (1 active-controlled trial) transplant patients.
Geriatrics
Elderly patients (≥65 years), particularly those who are receiving CellCept as part of a combination immunosuppressive regimen, may be at increased risk of certain infections (including cytomegalovirus [CMV] tissue invasive disease) and possibly gastrointestinal hemorrhage and pulmonary edema, compared to younger individuals (see PRECAUTIONS).
Safety data are summarized below for all active-controlled trials in renal (2 trials), cardiac (1 trial), and hepatic (1 trial) transplant patients. Approximately 53% of the renal patients, 65% of the cardiac patients, and 48% of the hepatic patients have been treated for more than 1 year. Adverse events reported in ≥20% of patients in the CellCept treatment groups are presented below.
Table 8 Adverse Events in Controlled Studies in Prevention of Renal, Cardiac or Hepatic Allograft Rejection (Reported in ≥20% of Patients in the CellCept Group) Renal Studies Cardiac Study Hepatic Study CellCept
2 g/dayCellCept
3 g/dayAzathioprine
1 to 2 mg/kg/day or 100 to 150 mg/dayCellCept
3 g/dayAzathioprine
1.5 to 3
mg/kg/dayCellCept
3 g/dayAzathioprine
1 to 2 mg/kg/day(n=336) (n=330) (n=326) (n=289) (n=289) (n=277) (n=287) % % % % % % % Body as a Whole Pain 33.0 31.2 32.2 75.8 74.7 74.0 77.7 Abdominal pain 24.7 27.6 23.0 33.9 33.2 62.5 51.2 Fever 21.4 23.3 23.3 47.4 46.4 52.3 56.1 Headache 21.1 16.1 21.2 54.3 51.9 53.8 49.1 Infection 18.2 20.9 19.9 25.6 19.4 27.1 25.1 Sepsis – – – – – 27.4 26.5 Asthenia – – – 43.3 36.3 35.4 33.8 Chest pain – – – 26.3 26.0 – – Back pain – – – 34.6 28.4 46.6 47.4 Ascites – – – – – 24.2 22.6 Hematologic and Lymphatic Anemia 25.6 25.8 23.6 42.9 43.9 43.0 53.0 Leukopenia 23.2 34.5 24.8 30.4 39.1 45.8 39.0 Thrombocytopenia – – – 23.5 27.0 38.3 42.2 Hypochromic anemia – – – 24.6 23.5 – – Leukocytosis – – – 40.5 35.6 22.4 21.3 Urogenital Urinary tract infection 37.2 37.0 33.7 – – – – Kidney function abnormal – – – 21.8 26.3 25.6 28.9 Cardiovascular Hypertension 32.4 28.2 32.2 77.5 72.3 62.1 59.6 Hypotension – – – 32.5 36.0 – – Cardiovascular disorder – – – 25.6 24.2 – – Tachycardia – – – 20.1 18.0 22.0 15.7 Metabolic and Nutritional Peripheral edema 28.6 27.0 28.2 64.0 53.3 48.4 47.7 Hyper-cholesteremia – – – 41.2 38.4 – – Edema – – – 26.6 25.6 28.2 28.2 Hypokalemia – – – 31.8 25.6 37.2 41.1 Hyperkalemia – – – – – 22.0 23.7 Hyperglycemia – – – 46.7 52.6 43.7 48.8 Creatinine increased – – – 39.4 36.0 – – BUN increased – – – 34.6 32.5 – – Lactic dehydrogenase increased – – – 23.2 17.0 – – Hypomagnesemia – – – – – 39.0 37.6 Hypocalcemia – – – – – 30.0 30.0 Digestive Diarrhea 31.0 36.1 20.9 45.3 34.3 51.3 49.8 Constipation 22.9 18.5 22.4 41.2 37.7 37.9 38.3 Nausea 19.9 23.6 24.5 54.0 54.3 54.5 51.2 Dyspepsia – – – – – 22.4 20.9 Vomiting – – – 33.9 28.4 32.9 33.4 Anorexia – – – – – 25.3 17.1 Liver function tests abnormal – – – – – 24.9 19.2 Respiratory Infection 22.0 23.9 19.6 37.0 35.3 – – Dyspnea – – – 36.7 36.3 31.0 30.3 Cough increased – – – 31.1 25.6 – – Lung disorder – – – 30.1 29.1 22.0 18.8 Sinusitis – – – 26.0 19.0 – – Pleural effusion – – – – – 34.3 35.9 Skin and Appendages Rash – – – 22.1 18.0 – – Nervous System Tremor – – – 24.2 23.9 33.9 35.5 Insomnia – – – 40.8 37.7 52.3 47.0 Dizziness – – – 28.7 27.7 – – Anxiety – – – 28.4 23.9 – – Paresthesia – – – 20.8 18.0 – – The placebo-controlled renal transplant study generally showed fewer adverse events occurring in ≥20% of patients. In addition, those that occurred were not only qualitatively similar to the azathioprine-controlled renal transplant studies, but also occurred at lower rates, particularly for infection, leukopenia, hypertension, diarrhea and respiratory infection.
The above data demonstrate that in three controlled trials for prevention of renal rejection, patients receiving 2 g/day of CellCept had an overall better safety profile than did patients receiving 3 g/day of CellCept.
The above data demonstrate that the types of adverse events observed in multicenter controlled trials in renal, cardiac, and hepatic transplant patients are qualitatively similar except for those that are unique to the specific organ involved.
Sepsis, which was generally CMV viremia, was slightly more common in renal transplant patients treated with CellCept compared to patients treated with azathioprine. The incidence of sepsis was comparable in CellCept and in azathioprine-treated patients in cardiac and hepatic studies.
In the digestive system, diarrhea was increased in renal and cardiac transplant patients receiving CellCept compared to patients receiving azathioprine, but was comparable in hepatic transplant patients treated with CellCept or azathioprine.
Patients receiving CellCept alone or as part of an immunosuppressive regimen are at increased risk of developing lymphomas and other malignancies, particularly of the skin (see WARNINGS: Lymphoma and Malignancy). The incidence of malignancies among the 1483 patients treated in controlled trials for the prevention of renal allograft rejection who were followed for ≥1 year was similar to the incidence reported in the literature for renal allograft recipients.
Lymphoproliferative disease or lymphoma developed in 0.4% to 1% of patients receiving CellCept (2 g or 3 g daily) with other immunosuppressive agents in controlled clinical trials of renal, cardiac, and hepatic transplant patients followed for at least 1 year (see WARNINGS: Lymphoma and Malignancy). Non-melanoma skin carcinomas occurred in 1.6% to 4.2% of patients, other types of malignancy in 0.7% to 2.1% of patients. Three-year safety data in renal and cardiac transplant patients did not reveal any unexpected changes in incidence of malignancy compared to the 1-year data.
In pediatric patients, no other malignancies besides lymphoproliferative disorder (2/148 patients) have been observed.
Severe neutropenia (ANC <0.5 × 103/µL) developed in up to 2.0% of renal transplant patients, up to 2.8% of cardiac transplant patients and up to 3.6% of hepatic transplant patients receiving CellCept 3 g daily (see WARNINGS: Neutropenia, PRECAUTIONS: Laboratory Tests and DOSAGE AND ADMINISTRATION).
All transplant patients are at increased risk of opportunistic infections. The risk increases with total immunosuppressive load (see WARNINGS: Infections and WARNINGS: Latent Viral Infections). Table 9 shows the incidence of opportunistic infections that occurred in the renal, cardiac, and hepatic transplant populations in the azathioprine-controlled prevention trials:
Table 9 Viral and Fungal Infections in Controlled Studies in Prevention of Renal, Cardiac or Hepatic Transplant Rejection Renal Studies Cardiac Study Hepatic Study CellCept
2 g/dayCellCept
3 g/dayAzathioprine
1 to 2 mg/kg/day or 100 to 150 mg/dayCellCept
3 g/dayAzathioprine
1.5 to 3 mg/kg/dayCellCept
3 g/dayAzathioprine
1 to 2 mg/kg/day(n=336) (n=330) (n=326) (n=289) (n=289) (n=277) (n=287) % % % % % % % Herpes simplex 16.7 20.0 19.0 20.8 14.5 10.1 5.9 CMV – Viremia/
syndrome13.4 12.4 13.8 12.1 10.0 14.1 12.2 – Tissue invasive disease 8.3 11.5 6.1 11.4 8.7 5.8 8.0 Herpes zoster 6.0 7.6 5.8 10.7 5.9 4.3 4.9 – Cutaneous disease 6.0 7.3 5.5 10.0 5.5 4.3 4.9 Candida 17.0 17.3 18.1 18.7 17.6 22.4 24.4 – Mucocutaneous 15.5 16.4 15.3 18.0 17.3 18.4 17.4 The following other opportunistic infections occurred with an incidence of less than 4% in CellCept patients in the above azathioprine-controlled studies: Herpes zoster, visceral disease; Candida, urinary tract infection, fungemia/disseminated disease, tissue invasive disease; Cryptococcosis; Aspergillus/Mucor; Pneumocystis carinii.
In the placebo-controlled renal transplant study, the same pattern of opportunistic infection was observed compared to the azathioprine-controlled renal studies, with a notably lower incidence of the following: Herpes simplex and CMV tissue-invasive disease.
In patients receiving CellCept (2 g or 3 g) in controlled studies for prevention of renal, cardiac or hepatic rejection, fatal infection/sepsis occurred in approximately 2% of renal and cardiac patients and in 5% of hepatic patients (see WARNINGS: Infections).
In cardiac transplant patients, the overall incidence of opportunistic infections was approximately 10% higher in patients treated with CellCept than in those receiving azathioprine, but this difference was not associated with excess mortality due to infection/sepsis among patients treated with CellCept.
The following adverse events were reported with 3% to <20% incidence in renal, cardiac, and hepatic transplant patients treated with CellCept, in combination with cyclosporine and corticosteroids.
Table 10 Adverse Events Reported in 3% to <20% of Patients Treated With CellCept in Combination With Cyclosporine and Corticosteroids Body System Body as a Whole abdomen enlarged, abscess, accidental injury, cellulitis, chills occurring with fever, cyst, face edema, flu syndrome, hemorrhage, hernia, lab test abnormal, malaise, neck pain, pelvic pain, peritonitis Hematologic and Lymphatic coagulation disorder, ecchymosis, pancytopenia, petechia, polycythemia, prothrombin time increased, thromboplastin time increased Urogenital acute kidney failure, albuminuria, dysuria, hydronephrosis, hematuria, impotence, kidney failure, kidney tubular necrosis, nocturia, oliguria, pain, prostatic disorder, pyelonephritis, scrotal edema, urine abnormality, urinary frequency, urinary incontinence, urinary retention, urinary tract disorder Cardiovascular angina pectoris, arrhythmia, arterial thrombosis, atrial fibrillation, atrial flutter, bradycardia, cardiovascular disorder, congestive heart failure, extrasystole, heart arrest, heart failure, hypotension, pallor, palpitation, pericardial effusion, peripheral vascular disorder, postural hypotension, pulmonary hypertension, supraventricular tachycardia, supraventricular extrasystoles, syncope, tachycardia, thrombosis, vasodilatation, vasospasm, ventricular extrasystole, ventricular tachycardia, venous pressure increased Metabolic and Nutritional abnormal healing, acidosis, alkaline phosphatase increased, alkalosis, bilirubinemia, creatinine increased, dehydration, gamma glutamyl transpeptidase increased, generalized edema, gout, hypercalcemia, hypercholesteremia, hyperlipemia, hyperphosphatemia, hyperuricemia, hypervolemia, hypocalcemia, hypochloremia, hypoglycemia, hyponatremia, hypophosphatemia, hypoproteinemia, hypovolemia, hypoxia, lactic dehydrogenase increased, respiratory acidosis, SGOT increased, SGPT increased, thirst, weight gain, weight loss Digestive anorexia, cholangitis, cholestatic jaundice, dysphagia, esophagitis, flatulence, gastritis, gastroenteritis, gastrointestinal disorder, gastrointestinal hemorrhage, gastrointestinal moniliasis, gingivitis, gum hyperplasia, hepatitis, ileus, infection, jaundice, liver damage, liver function tests abnormal, melena, mouth ulceration, nausea and vomiting, oral moniliasis, rectal disorder, stomach ulcer, stomatitis Respiratory apnea, asthma, atelectasis, bronchitis, epistaxis, hemoptysis, hiccup, hyperventilation, lung edema, lung disorder, neoplasm, pain, pharyngitis, pleural effusion, pneumonia, pneumothorax, respiratory disorder, respiratory moniliasis, rhinitis, sinusitis, sputum increased, voice alteration Skin and Appendages acne, alopecia, fungal dermatitis, hemorrhage, hirsutism, pruritus, rash, skin benign neoplasm, skin carcinoma, skin disorder, skin hypertrophy, skin ulcer, sweating, vesiculobullous rash Nervous agitation, anxiety, confusion, convulsion, delirium, depression, dry mouth, emotional lability, hallucinations, hypertonia, hypesthesia, nervousness, neuropathy, paresthesia, psychosis, somnolence, thinking abnormal, vertigo Endocrine Cushing's syndrome, diabetes mellitus, hypothyroidism, parathyroid disorder Musculoskeletal arthralgia, joint disorder, leg cramps, myalgia, myasthenia, osteoporosis Special Senses abnormal vision, amblyopia, cataract (not specified), conjunctivitis, deafness, ear disorder, ear pain, eye hemorrhage, tinnitus, lacrimation disorder Pediatrics
The type and frequency of adverse events in a clinical study in 100 pediatric patients 3 months to 18 years of age dosed with CellCept oral suspension 600 mg/m2 bid (up to 1 g bid) were generally similar to those observed in adult patients dosed with CellCept capsules at a dose of 1 g bid with the exception of abdominal pain, fever, infection, pain, sepsis, diarrhea, vomiting, pharyngitis, respiratory tract infection, hypertension, leukopenia, and anemia, which were observed in a higher proportion in pediatric patients.
CellCept Intravenous
The adverse event profile of CellCept Intravenous was determined from a single, double-blind, controlled comparative study of the safety of 2 g/day of intravenous and oral CellCept in renal transplant patients in the immediate posttransplant period (administered for the first 5 days). The potential venous irritation of CellCept Intravenous was evaluated by comparing the adverse events attributable to peripheral venous infusion of CellCept Intravenous with those observed in the intravenous placebo group; patients in this group received active medication by the oral route.
Adverse events attributable to peripheral venous infusion were phlebitis and thrombosis, both observed at 4% in patients treated with CellCept Intravenous.
In the active controlled study in hepatic transplant patients, 2 g/day of CellCept Intravenous were administered in the immediate posttransplant period (up to 14 days). The safety profile of intravenous CellCept was similar to that of intravenous azathioprine.
Postmarketing Experience
Congenital Disorders
Congenital malformations including ear malformations have been reported in offspring of patients exposed to mycophenolate mofetil during pregnancy (see WARNINGS: Pregnancy).
Digestive
Colitis (sometimes caused by cytomegalovirus), pancreatitis, isolated cases of intestinal villous atrophy.
Hematologic and Lymphatic
Cases of pure red cell aplasia (PRCA) have been reported in patients treated with CellCept in combination with other immunosuppressive agents.
Infections
Serious life-threatening infections such as meningitis and infectious endocarditis have been reported occasionally and there is evidence of a higher frequency of certain types of serious infections such as tuberculosis and atypical mycobacterial infection. Cases of progressive multifocal leukoencephalopathy (PML), sometimes fatal, have been reported in patients treated with CellCept. The reported cases generally had risk factors for PML, including treatment with immunosuppressant therapies and impairment of immune function. BK virus-associated nephropathy has been observed in patients receiving immunosuppressants, including CellCept. This infection is associated with serious outcomes, including deteriorating renal function and renal graft loss.
-
OVERDOSAGE
The experience with overdose of CellCept in humans is very limited. The events received from reports of overdose fall within the known safety profile of the drug. The highest dose administered to renal transplant patients in clinical trials has been 4 g/day. In limited experience with cardiac and hepatic transplant patients in clinical trials, the highest doses used were 4 g/day or 5 g/day. At doses of 4 g/day or 5 g/day, there appears to be a higher rate, compared to the use of 3 g/day or less, of gastrointestinal intolerance (nausea, vomiting, and/or diarrhea), and occasional hematologic abnormalities, principally neutropenia, leading to a need to reduce or discontinue dosing.
In acute oral toxicity studies, no deaths occurred in adult mice at doses up to 4000 mg/kg or in adult monkeys at doses up to 1000 mg/kg; these were the highest doses of mycophenolate mofetil tested in these species. These doses represent 11 times the recommended clinical dose in renal transplant patients and approximately 7 times the recommended clinical dose in cardiac transplant patients when corrected for BSA. In adult rats, deaths occurred after single-oral doses of 500 mg/kg of mycophenolate mofetil. The dose represents approximately 3 times the recommended clinical dose in cardiac transplant patients when corrected for BSA.
MPA and MPAG are usually not removed by hemodialysis. However, at high MPAG plasma concentrations (>100 µg/mL), small amounts of MPAG are removed. By increasing excretion of the drug, MPA can be removed by bile acid sequestrants, such as cholestyramine (see CLINICAL PHARMACOLOGY: Pharmacokinetics).
-
DOSAGE AND ADMINISTRATION
Renal Transplantation
Adults
A dose of 1 g administered orally or intravenously (over NO LESS THAN 2 HOURS) twice a day (daily dose of 2 g) is recommended for use in renal transplant patients. Although a dose of 1.5 g administered twice daily (daily dose of 3 g) was used in clinical trials and was shown to be safe and effective, no efficacy advantage could be established for renal transplant patients. Patients receiving 2 g/day of CellCept demonstrated an overall better safety profile than did patients receiving 3 g/day of CellCept.
Pediatrics (3 months to 18 years of age)
The recommended dose of CellCept oral suspension is 600 mg/m2 administered twice daily (up to a maximum daily dose of 2 g/10 mL oral suspension). Patients with a body surface area of 1.25 m2 to 1.5 m2 may be dosed with CellCept capsules at a dose of 750 mg twice daily (1.5 g daily dose). Patients with a body surface area >1.5 m2 may be dosed with CellCept capsules or tablets at a dose of 1 g twice daily (2 g daily dose).
CellCept Capsules, Tablets, and Oral Suspension
The initial oral dose of CellCept should be given as soon as possible following renal, cardiac or hepatic transplantation. Food had no effect on MPA AUC, but has been shown to decrease MPA Cmax by 40%. Therefore, it is recommended that CellCept be administered on an empty stomach. However, in stable renal transplant patients, CellCept may be administered with food if necessary.
Note:
If required, CellCept Oral Suspension can be administered via a nasogastric tube with a minimum size of 8 French (minimum 1.7 mm interior diameter).Patients With Hepatic Impairment
No dose adjustments are recommended for renal patients with severe hepatic parenchymal disease. However, it is not known whether dose adjustments are needed for hepatic disease with other etiologies (see CLINICAL PHARMACOLOGY: Pharmacokinetics).
No data are available for cardiac transplant patients with severe hepatic parenchymal disease.
Geriatrics
The recommended oral dose of 1 g bid for renal transplant patients, 1.5 g bid for cardiac transplant patients, and 1 g bid administered intravenously or 1.5 g bid administered orally in hepatic transplant patients is appropriate for elderly patients (see PRECAUTIONS: Geriatric Use).
Preparation of Oral Suspension
It is recommended that CellCept Oral Suspension be constituted by the pharmacist prior to dispensing to the patient.
CellCept Oral Suspension should not be mixed with any other medication.
Mycophenolate mofetil has demonstrated teratogenic effects in rats and rabbits. There are no adequate and well-controlled studies in pregnant women (see WARNINGS, PRECAUTIONS, ADVERSE REACTIONS, and HANDLING AND DISPOSAL). Care should be taken to avoid inhalation or direct contact with skin or mucous membranes of the dry powder or the constituted suspension. If such contact occurs, wash thoroughly with soap and water; rinse eyes with water.
- Tap the closed bottle several times to loosen the powder.
- Measure 94 mL of water in a graduated cylinder.
- Add approximately half the total amount of water for constitution to the bottle and shake the closed bottle well for about 1 minute.
- Add the remainder of water and shake the closed bottle well for about 1 minute.
- Remove the child-resistant cap and push bottle adapter into neck of bottle.
- Close bottle with child-resistant cap tightly. This will assure the proper seating of the bottle adapter in the bottle and child-resistant status of the cap.
Dispense with patient instruction sheet and oral dispensers. It is recommended to write the date of expiration of the constituted suspension on the bottle label. (The shelf-life of the constituted suspension is 60 days.)
After constitution the oral suspension contains 200 mg/mL mycophenolate mofetil. Store constituted suspension at 25°C (77°F); excursions permitted to 15° to 30°C (59° to 86°F). Storage in a refrigerator at 2° to 8°C (36° to 46°F) is acceptable. Do not freeze. Discard any unused portion 60 days after constitution.
CellCept Intravenous
Adults
CellCept Intravenous is an alternative dosage form to CellCept capsules, tablets and oral suspension recommended for patients unable to take oral CellCept. CellCept Intravenous should be administered within 24 hours following transplantation. CellCept Intravenous can be administered for up to 14 days; patients should be switched to oral CellCept as soon as they can tolerate oral medication.
CellCept Intravenous must be reconstituted and diluted to a concentration of 6 mg/mL using 5% Dextrose Injection USP. CellCept Intravenous is incompatible with other intravenous infusion solutions. Following reconstitution, CellCept Intravenous must be administered by slow intravenous infusion over a period of NO LESS THAN 2 HOURS by either peripheral or central vein.
CAUTION: CELLCEPT INTRAVENOUS SOLUTION SHOULD NEVER BE ADMINISTERED BY RAPID OR BOLUS INTRAVENOUS INJECTION (see WARNINGS).
Preparation of Infusion Solution (6 mg/mL)
Caution should be exercised in the handling and preparation of solutions of CellCept Intravenous. Avoid direct contact of the prepared solution of CellCept Intravenous with skin or mucous membranes. If such contact occurs, wash thoroughly with soap and water; rinse eyes with plain water (see WARNINGS, PRECAUTIONS, ADVERSE REACTIONS, and HANDLING AND DISPOSAL).
CellCept Intravenous does not contain an antibacterial preservative; therefore, reconstitution and dilution of the product must be performed under aseptic conditions. Additionally, this product is sealed under vacuum and should retain a vacuum throughout its shelf life. If a lack of vacuum in the vial is noted while adding diluent, the vial should not be used.
CellCept Intravenous infusion solution must be prepared in two steps: the first step is a reconstitution step with 5% Dextrose Injection USP, and the second step is a dilution step with 5% Dextrose Injection USP. A detailed description of the preparation is given below:
Step 1
- a)Two (2) vials of CellCept Intravenous are used for preparing each 1 g dose, whereas three (3) vials are needed for each 1.5 g dose. Reconstitute the contents of each vial by injecting 14 mL of 5% Dextrose Injection USP.
- b)Gently shake the vial to dissolve the drug.
- c)Inspect the resulting slightly yellow solution for particulate matter and discoloration prior to further dilution. Discard the vials if particulate matter or discoloration is observed.
Step 2
- a)To prepare a 1 g dose, further dilute the contents of the two reconstituted vials (approx. 2 × 15 mL) into 140 mL of 5% Dextrose Injection USP. To prepare a 1.5 g dose, further dilute the contents of the three reconstituted vials (approx. 3 × 15 mL) into 210 mL of 5% Dextrose Injection USP. The final concentration of both solutions is 6 mg mycophenolate mofetil per mL.
- b)Inspect the infusion solution for particulate matter or discoloration. Discard the infusion solution if particulate matter or discoloration is observed.
If the infusion solution is not prepared immediately prior to administration, the commencement of administration of the infusion solution should be within 4 hours from reconstitution and dilution of the drug product. Keep solutions at 25°C (77°F); excursions permitted to 15° to 30°C (59° to 86°F).
CellCept Intravenous should not be mixed or administered concurrently via the same infusion catheter with other intravenous drugs or infusion admixtures.
Dosage Adjustments
In renal transplant patients with severe chronic renal impairment (GFR <25 mL/min/1.73 m2) outside the immediate posttransplant period, doses of CellCept greater than 1 g administered twice a day should be avoided. These patients should also be carefully observed. No dose adjustments are needed in renal transplant patients experiencing delayed graft function postoperatively (see CLINICAL PHARMACOLOGY: Pharmacokinetics and PRECAUTIONS: General).
No data are available for cardiac or hepatic transplant patients with severe chronic renal impairment. CellCept may be used for cardiac or hepatic transplant patients with severe chronic renal impairment if the potential benefits outweigh the potential risks.
If neutropenia develops (ANC <1.3 × 103/µL), dosing with CellCept should be interrupted or the dose reduced, appropriate diagnostic tests performed, and the patient managed appropriately (see WARNINGS: Neutropenia, ADVERSE REACTIONS, and PRECAUTIONS: Laboratory Tests).
-
HANDLING AND DISPOSAL
Mycophenolate mofetil has demonstrated teratogenic effects in rats and rabbits (see WARNINGS: Pregnancy). CellCept tablets should not be crushed and CellCept capsules should not be opened or crushed. Avoid inhalation or direct contact with skin or mucous membranes of the powder contained in CellCept capsules and CellCept Oral Suspension (before or after constitution). If such contact occurs, wash thoroughly with soap and water; rinse eyes with plain water. Should a spill occur, wipe up using paper towels wetted with water to remove spilled powder or suspension. Caution should be exercised in the handling and preparation of solutions of CellCept Intravenous. Avoid direct contact of the prepared solution of CellCept Intravenous with skin or mucous membranes. If such contact occurs, wash thoroughly with soap and water; rinse eyes with plain water.
-
HOW SUPPLIED
CellCept (mycophenolate mofetil capsules) 250 mg
Blue-brown, two-piece hard gelatin capsules, printed in black with "CellCept 250" on the blue cap and "Roche" on the brown body. Supplied in the following presentations:
NDC Number Size NDC: 21695-171-00 Bottle of 100 -
MEDICATION GUIDE
CellCept® [SEL-sept]
(mycophenolate mofetil capsules)
(mycophenolate mofetil tablets)CellCept® Oral Suspension
(mycophenolate mofetil for oral suspension)CellCept® Intravenous
(mycophenolate mofetil hydrochloride for injection)Read the Medication Guide that comes with CellCept before you start taking it and each time you refill your prescription. There may be new information. This Medication Guide does not take the place of talking with your healthcare provider about your medical condition or your treatment.
What is the most important information I should know about CellCept?
CellCept can cause serious side effects:
-
Possible loss of a pregnancy and higher risk of birth defects. Women who take CellCept during pregnancy have a higher risk of losing a pregnancy (miscarriage) during the first 3 months (first trimester), and a higher risk that their baby will be born with birth defects
If you are a female and are able to become pregnant- your healthcare provider must talk with you about effective birth control methods (contraceptive counseling)
- you should have a negative pregnancy test within 1 week before you start to take CellCept
- you must use 2 different types of effective birth control at the same time, for 4 weeks before you start taking CellCept, during your entire CellCept therapy and for 6 weeks after stopping CellCept, unless you choose to avoid sexual intercourse completely (abstinence). CellCept decreases blood levels of the hormones in birth control pills that you take by mouth. Birth control pills may not work as well while you take CellCept, and you could become pregnant
If you plan to become pregnant, talk with your healthcare provider. Your healthcare provider will decide if other medicines to prevent rejection may be right for you. In certain situations, you and your healthcare provider may decide that taking CellCept is more important to your health than the possible risks to your unborn baby.
-
If you get pregnant while taking CellCept, do not stop taking CellCept. Call your healthcare provider right away. You and your healthcare provider should report any cases of pregnancies to
- FDA MedWatch at 1-800-FDA-1088
- Genentech at 1-888-835-2555
Talk to your healthcare provider about joining the National Transplantation Pregnancy Registry at 1-877-955-6877.
-
Increased risk of getting serious infections. CellCept weakens the body's immune system and affects your ability to fight infections. Serious infections can happen with CellCept and can lead to death. Types of infections can include:
-
Viral infections. Certain viruses can live in your body and cause active infections when your immune system is weak. Viral infections that can happen with CellCept include:
- Shingles, other herpes infections, and cytomegalovirus (CMV). CMV can cause serious tissue and blood infections.
- BK virus. BK virus can affect how your kidney works and cause your transplanted kidney to fail.
-
A brain infection called Progressive Multifocal Leukoencephalopathy (PML). In some patients, CellCept may cause an infection of the brain that may cause death. You are at risk for this brain infection because you have a weakened immune system. You should tell your healthcare provider right away if you have any of the following symptoms:
- Weakness on one side of the body
- You do not care about things that you usually care about (apathy)
- You are confused or have problems thinking
- You can not control your muscles
- Fungal infections. Yeasts and other types of fungal infections can happen with CellCept and can cause serious tissue and blood infections (see "What are the possible side effects of CellCept?")
-
Viral infections. Certain viruses can live in your body and cause active infections when your immune system is weak. Viral infections that can happen with CellCept include:
Call your healthcare provider right away if you have any of the following signs and symptoms of infection:
- Temperature of 100.5°F or greater
- Cold symptoms, such as a runny nose or sore throat
- Flu symptoms, such as an upset stomach, stomach pain, vomiting or diarrhea
- Earache or headache
- Pain during urination
- White patches in the mouth or throat
- Unexpected bruising or bleeding
- Cuts, scrapes or incisions that are red, warm and oozing pus
-
Increased risk of getting certain cancers. People who take CellCept have a higher risk of getting lymphoma, and other cancers, especially skin cancer. Tell your healthcare provider if you have:
- unexplained fever, prolonged tiredness, weight loss or lymph node swelling
- a brown or black skin lesion with uneven borders, or one part of the lesion does not look like the other
- a change in the size and color of a mole
- a new skin lesion or bump
- any other changes to your health
See the section "What are the possible side effects of CellCept?" for information about other serious side effects.
What is CellCept?
CellCept is a prescription medicine to prevent rejection (antirejection medicine) in people who have received a kidney, heart or liver transplant. Rejection is when the body's immune system perceives the new organ as a "foreign" threat and attacks it.
CellCept is used with other medicines called cyclosporine (Sandimmune®, Gengraf®, Neoral®) and corticosteroids. These medicines work together to prevent rejection to your transplanted organ.
CellCept has been used safely and works in children who received a kidney transplant as it does in adults. It is not known if CellCept is safe and works in children who receive a heart or liver transplant.
Who should not take CellCept?
Do not take CellCept if you are allergic to mycophenolate mofetil or any of the ingredients in CellCept. See the end of this Medication Guide for a complete list of ingredients in CellCept.
What should I tell my healthcare provider before taking CellCept?
Tell your healthcare provider about all of your medical conditions, if you:
- have any digestive problems, such as ulcers
- have Phenylketonuria (PKU). CellCept oral suspension contains aspartame (a source of phenylalanine)
- have Lesch-Nyhan or Kelley-Seegmiller syndrome or another rare inherited deficiency hypoxanthine-guanine phosphoribosyl-transferase (HGPRT). You should not take CellCept if you have one of these disorders
- plan to receive any vaccines. People taking CellCept should not take live vaccines. Some vaccines may not work as well during treatment with CellCept
- are pregnant or are planning to become pregnant. See "What is the most important information I should know about CellCept?"
- are breastfeeding. It is not known if CellCept passes into breast milk. You and your healthcare provider will decide if you will take CellCept or breastfeed. You should not do both without first talking with your healthcare provider
Tell your healthcare provider about all of the medicines you are taking including prescription and nonprescription medicines, vitamins and herbal supplements. Some medicines may affect the way CellCept works, and CellCept may affect how some medicines work. Especially tell your healthcare provider if you take:
- birth control pills (oral contraceptives). See "What is the most important information I should know about CellCept?"
- sevelamer (Renagel®, Renvela™). These products should be taken 2 hours after taking CellCept
- acyclovir (Zovirax®), valacyclovir (Valtrex®), ganciclovir (CYTOVENE®-IV, Vitrasert®), valganciclovir (VALCYTE®)
- rifampin (Rifater®, Rifamate®, Rimactane®, Rifadin®)
- antacids that contain magnesium and aluminum (CellCept and the antacid should not be taken at the same time)
- sulfamethoxazole/trimethoprim (BACTRIM™, BACTRIM DS™)
- norfloxacin (Noroxin®) and metronidazole (Flagyl®, Flagyl® ER, Flagyl® IV, Metro IV, Helidac®, Pylera™)
- ciprofloxacin (Cipro®, Cipro® XR, Ciloxan®, Proquin® XR) and amoxicillin plus clavulanic acid (Augmentin®, Augmentin XR™)
- azathioprine (Azasan®, Imuran®)
- cholestyramine (Questran Light®, Questran®, Locholest Light, Locholest, Prevalite®)
Know the medicines you take. Keep a list of them to show to your healthcare provider and pharmacist when you get a new medicine. Do not take any new medicine without talking with your healthcare provider.
How should I take CellCept?
- Take CellCept exactly as prescribed
- Do not stop taking CellCept or change the dose unless your healthcare provider tells you to
- If you miss a dose of CellCept, or are not sure when you took your last dose, take the regular amount of CellCept prescribed as soon as you remember. If it is time for your next dose, skip the missed dose. Do not take 2 doses at the same time. Call your healthcare provider if you are not sure what to do
- Take CellCept capsules, tablets and oral suspension on an empty stomach, either 1 hour before or 2 hours after a meal, unless your healthcare provider tells you otherwise. With the approval of your healthcare provider, in stable kidney transplant patients, CellCept can be taken with food if necessary
- Most people take CellCept by mouth either as blue and brown capsules or lavender tablets. Some people may get CellCept soon after their transplant surgery as an infusion into a vein
- Do not crush CellCept tablets. Do not open or crush CellCept capsules
- If you are not able to swallow CellCept tablets or capsules, your healthcare provider may prescribe CellCept Oral Suspension. This is a liquid form of CellCept. Your pharmacist will mix the medicine before giving it to you
- Do not mix CellCept Oral Suspension with any other medicine
- If you take too much CellCept, call your healthcare provider or the poison control center right away
What should I avoid while taking CellCept?
- Avoid pregnancy. See "What is the most important information I should know about CellCept?"
- Limit the amount of time you spend in sunlight. Avoid using tanning beds or sunlamps. People who take CellCept have a higher risk of getting skin cancer. (See "What is the most important information I should know about CellCept?") Wear protective clothing when you are in the sun and use a sunscreen with a high protection factor (SPF 30 and above). This is especially important if your skin is very fair or if you have a family history of skin cancer
What are the possible side effects of CellCept?
CellCept can cause serious side effects:
- See "What is the most important information I should know about CellCept?"
-
Low blood cell counts. People taking high doses of CellCept each day may have a decrease in blood counts, including
- white blood cells, especially neutrophils. Neutrophils fight against bacterial infections. You have a higher chance of getting an infection when your white blood cell count is low. This is most common from 3 months to 6 months after your transplant
- red blood cells. Red blood cells carry oxygen to your body tissues. You have a higher chance of getting severe anemia when your red blood cell count is low
- platelets. Platelets help with blood clotting
Your healthcare provider will do blood tests before you start taking CellCept and during treatment with CellCept to check your blood cell counts.
Tell your healthcare provider right away if you have any signs of infection (see "What is the most important information I should know about CellCept?"), or any unexpected bruising or bleeding. Also, tell your healthcare provider if you have unusual tiredness, lack of energy, dizziness or fainting. - Stomach problems. Stomach and intestinal bleeding can happen in people who take high doses of CellCept. Bleeding can be severe and you may have to be hospitalized for treatment
Common side effects include:
- diarrhea. Call your healthcare provider right away if you have diarrhea. Do not stop taking CellCept without first talking with your healthcare provider
- vomiting
- pain
- stomach area pain
- swelling of the lower legs, ankles and feet
- high blood pressure
Side effects that happen more often in children than in adults taking CellCept include:
- stomach area pain
- fever
- infection
- pain
- blood infection (sepsis)
- diarrhea
- vomiting
- sore throat
- colds (respiratory tract infections)
- high blood pressure
- low white blood cell count
- low red blood cell count
These are not all of the possible side effects of CellCept. Tell your healthcare provider about any side effect that bothers you or that does not go away.
Call your doctor for medical advice about side effects. You may report side effects to the FDA at 1-800-FDA-1088 or to Genentech at 1-888-835-2555.
How should I store CellCept?
- Store CellCept capsules and tablets at room temperature, between 59°F to 86°F (15°C to 30°C). Keep the container closed tightly
- Store the prepared CellCept Oral Suspension at room temperature, between 59°F to 86°F (15°C to 30°C), for up to 60 days. You can also store CellCept Oral Suspension in the refrigerator at 36°F to 46°F (2°C to 8°C). Do not freeze CellCept Oral Suspension
- Keep CellCept and all medicines out of the reach of children
General Information about CellCept
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use CellCept for a condition for which it was not prescribed. Do not give CellCept to other people, even if they have the same symptoms that you have. It may harm them.
This Medication Guide summarizes the most important information about CellCept. If you would like more information, talk with your doctor. You can ask your doctor or pharmacist for information about CellCept that is written for healthcare professionals. For more information, call 1-888-835-2555 or visit www.gene.com/gene/products/information/cellcept.
What are the ingredients in CellCept?
Active Ingredient: mycophenolate mofetil
Inactive Ingredients:
CellCept 250 mg capsules: croscarmellose sodium, magnesium stearate, povidone (K-90) and pregelatinized starch. The capsule shells contain black iron oxide, FD&C blue #2, gelatin, red iron oxide, silicon dioxide, sodium lauryl sulfate, titanium dioxide, and yellow iron oxide.
CellCept 500 mg tablets: black iron oxide, croscarmellose sodium, FD&C blue #2 aluminum lake, hydroxypropyl cellulose, hydroxypropyl methylcellulose, magnesium stearate, microcrystalline cellulose, polyethylene glycol 400, povidone (K-90), red iron oxide, talc, and titanium dioxide; may also contain ammonium hydroxide, ethyl alcohol, methyl alcohol, n-butyl alcohol, propylene glycol, and shellac.
CellCept Oral Suspension: aspartame, citric acid anhydrous, colloidal silicon dioxide, methylparaben, mixed fruit flavor, sodium citrate dihydrate, sorbitol, soybean lecithin, and xanthan gum.
CellCept Intravenous: polysorbate 80, and citric acid. Sodium hydroxide may have been used in the manufacture of CellCept Intravenous to adjust the pH.
This Medication Guide has been approved by the US Food and Drug Administration.
CELLCEPT, CYTOVENE-IV, and VALCYTE are registered trademarks of Hoffmann-La Roche Inc.
BACTRIM and BACTRIM DS are trademarks of Hoffmann-La Roche Inc.Distributed by:
Genentech USA, Inc.
A Member of the Roche Group
1 DNA Way
South San Francisco, CA 94080-4990Repackaged by:
Rebel Distributors Corp
Thousand Oaks, CA 91320
MG Revised: February 2010
For additional copies of this Medication Guide, please call 1-800-617-8191 or visit www.gene.com/gene/products/information/cellcept.
CTCTIO_1061443_PI/MG_AR2010_N(2)
CTCTIO_1061443_PI/MG_AR2010_K(2)© 2010 Genentech, Inc. All rights reserved.
Representative sample of labeling (see the HOW SUPPLIED section for complete listing):
-
Possible loss of a pregnancy and higher risk of birth defects. Women who take CellCept during pregnancy have a higher risk of losing a pregnancy (miscarriage) during the first 3 months (first trimester), and a higher risk that their baby will be born with birth defects
-
PRINCIPAL DISPLAY PANEL - 250 mg Capsule Carton
NDC: 21695-171-00
CellCept®
(mycophenolate
mofetil capsules)250 mg
Each capsule contains
250 mg mycophenolate mofetil.Rx only
Attention Pharmacist: Dispense the
accompanying Medication Guide to each
patient. For additional Medication Guides
call 1-800-617-8191 or visit
www.gene.com/gene/
products/information/cellcept.100 capsules
Rebel Distributors Corp
-
INGREDIENTS AND APPEARANCE
CELLCEPT
mycophenolate mofetil capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 21695-171(NDC: 59643-259) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Mycophenolate Mofetil (UNII: 9242ECW6R0) (mycophenolic acid - UNII:HU9DX48N0T) Mycophenolate Mofetil 250 mg Inactive Ingredients Ingredient Name Strength croscarmellose sodium (UNII: M28OL1HH48) magnesium stearate (UNII: 70097M6I30) Povidone K90 (UNII: RDH86HJV5Z) starch, corn (UNII: O8232NY3SJ) ferrosoferric oxide (UNII: XM0M87F357) FD&C blue No. 2 (UNII: L06K8R7DQK) gelatin (UNII: 2G86QN327L) ferric oxide yellow (UNII: EX438O2MRT) ferric oxide red (UNII: 1K09F3G675) titanium dioxide (UNII: 15FIX9V2JP) silicon dioxide (UNII: ETJ7Z6XBU4) sodium lauryl sulfate (UNII: 368GB5141J) Product Characteristics Color BLUE, BROWN Score no score Shape CAPSULE Size 19mm Flavor Imprint Code CellCept;250;Roche Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 21695-171-00 100 in 1 BOTTLE, PLASTIC Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA050722 05/03/1995 Labeler - Rebel Distributors Corp (118802834) Establishment Name Address ID/FEI Business Operations Rebel Distributors Corp 118802834 RELABEL, REPACK
Trademark Results [CellCept]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 CELLCEPT 74357234 1889130 Live/Registered |
Hoffmann-La Roche Inc 1993-02-05 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.