These highlights do not include all the information needed to use SPIKEVAX safely and effectively. See full prescribing information for SPIKEVAX. SPIKEVAX (COVID-19 Vaccine, mRNA) Suspension for injection, for intramuscular use Initial U.S. Approval: 2022
Spikevax by
Drug Labeling and Warnings
Spikevax by is a Other medication manufactured, distributed, or labeled by Moderna US, Inc., ModernaTX, Inc., Lonza Biologics, Inc., Catalent Indiana, LLC, Baxter Pharmaceutical Solutions, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
SPIKEVAX- covid-19 vaccine, mrna injection, suspension
Moderna US, Inc.
----------
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use SPIKEVAX safely and effectively. See full prescribing information for SPIKEVAX.
SPIKEVAX (COVID-19 Vaccine, mRNA) Suspension for injection, for intramuscular use Initial U.S. Approval: 2022 INDICATIONS AND USAGESPIKEVAX is a vaccine indicated for active immunization to prevent coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in individuals 18 years of age and older. (1) DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHSSuspension for injection. A single dose is 0.5 mL. (3) CONTRAINDICATIONSSevere allergic reaction (e.g., anaphylaxis) to any component of SPIKEVAX. (4) WARNINGS AND PRECAUTIONSPostmarketing data demonstrate increased risks of myocarditis and pericarditis, particularly within 7 days following the second dose. (5.2) ADVERSE REACTIONS
To report SUSPECTED ADVERSE REACTIONS, contact ModernaTX, Inc. at 1-866-663-3762 or VAERS at 1-800-822-7967 or http://vaers.hhs.gov. See 17 for PATIENT COUNSELING INFORMATION. Revised: 9/2023 |
FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
SPIKEVAX is a vaccine indicated for active immunization to prevent coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in individuals 18 years of age and older.
2 DOSAGE AND ADMINISTRATION
For intramuscular injection only.
2.1 Preparation for Administration
-
SPIKEVAX is supplied in two presentations:
- o multiple-dose vial containing 5.5 mL
- o multiple-dose vial containing 7.5 mL
- SPIKEVAX multiple-dose vials contain a frozen suspension that does not contain a preservative and must be thawed prior to administration.
- Thaw each vial before use following the instructions below.
|
Multiple-Dose Vial Containing |
Thaw in Refrigerator |
Thaw at Room Temperature |
|
5.5 mL |
Thaw between 2°C to 8°C (36°F to 46°F) for 2 hours and 30 minutes. Let each vial stand at room temperature for 15 minutes before administering. |
Alternatively, thaw between 15°C to 25°C (59°F to 77°F) for 1 hour. |
|
7.5 mL |
Thaw between 2°C to 8°C (36°F to 46°F) for 3 hours. Let each vial stand at room temperature for 15 minutes before administering. |
Alternatively, thaw between 15°C to 25°C (59°F to 77°F) for 1 hour and 30 minutes. |
- After thawing, do not refreeze.
- Swirl vial gently after thawing and between each withdrawal. Do not shake. Do not dilute the vaccine.
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
- SPIKEVAX is a white to off-white suspension. It may contain white or translucent product-related particulates. Do not administer if vaccine is discolored or contains other particulate matter.
- Each dose is 0.5 mL.
- If the amount of vaccine remaining in the vial cannot provide a full dose of 0.5 mL, discard the vial and contents. Do not pool excess vaccine from multiple vials.
- After the first dose has been withdrawn, the vial should be held between 2°C to 25°C (36°F to 77°F). Record the date and time of first use on the SPIKEVAX vial label. Discard vial after 12 hours. Do not refreeze.
2.3 Dosing and Schedule
SPIKEVAX is administered intramuscularly as a series of two doses (0.5 mL each) 1 month apart.
There are no data available on the interchangeability of SPIKEVAX with COVID-19 vaccines from other manufacturers to complete the vaccination series. Individuals who have received one dose of SPIKEVAX should receive a second dose of SPIKEVAX to complete the vaccination series.
4 CONTRAINDICATIONS
Do not administer SPIKEVAX to individuals with a known history of severe allergic reaction (e.g., anaphylaxis) to any component of SPIKEVAX [see Description (11)].
5 WARNINGS AND PRECAUTIONS
5.1 Management of Acute Allergic Reactions
Appropriate medical treatment to manage immediate allergic reactions must be immediately available in the event an acute anaphylactic reaction occurs following administration of SPIKEVAX.
5.2 Myocarditis and Pericarditis
Postmarketing data demonstrate increased risks of myocarditis and pericarditis, particularly within 7 days following the second dose. The observed risk is higher among males under 40 years of age than among females and older males. The observed risk is highest in males 18 through 24 years of age. Although some cases required intensive care support, available data from short-term follow-up suggest that most individuals have had resolution of symptoms with conservative management. Information is not yet available about potential long-term sequelae. The CDC has published considerations related to myocarditis and pericarditis after vaccination, including for vaccination of individuals with a history of myocarditis or pericarditis (https://www.cdc.gov/vaccines/covid-19/clinical-considerations/myocarditis.html).
5.3 Syncope
Syncope (fainting) may occur in association with administration of injectable vaccines including SPIKEVAX. Procedures should be in place to avoid injury from fainting.
6 ADVERSE REACTIONS
In study participants 18 through 64 years of age, the most commonly reported (≥10%) adverse reactions following any dose were pain at injection site (93.3%), fatigue (71.9%), headache (68.7%), myalgia (64.8%), chills (49.7%), arthralgia (48.6%), nausea/vomiting (25.7%), axillary swelling/tenderness (22.2%), fever (17.3%), swelling at the injection site (15.4%), and erythema at the injection site (10.5%).
In study participants 65 years of age and older, the most commonly reported (≥10%) adverse reactions following any dose were pain at injection site (88.3%), fatigue (64.8%), headache (53.3%), myalgia (51.8%), arthralgia (40.2%), chills (32.7%), nausea/vomiting (15.0%), swelling at the injection site (13.0%), and axillary swelling/tenderness (12.7%).
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a vaccine cannot be directly compared with rates in the clinical trials of another vaccine and may not reflect the rates observed in practice.
The safety of SPIKEVAX was evaluated in an ongoing Phase 3 randomized, placebo-controlled, observer-blind clinical trial conducted in the United States involving 30,346 participants 18 years of age and older who received at least one dose of SPIKEVAX (n=15,184) or placebo (n=15,162) (Study 1, NCT04470427). Upon issuance of the Emergency Use Authorization (December 18, 2020) for Moderna COVID-19 Vaccine (SPIKEVAX), participants were unblinded in a phased manner over a period of months to offer placebo participants SPIKEVAX. The median duration of follow up for safety after the second injection during the blinded phase was 4 months. The median duration of follow up for safety after the second injection including both the blinded phase and the open-label phase was 6 months.
In Study 1, the median age of the population was 52 years (range 18-95); 22,826 (75.2%) participants were 18 to 64 years of age and 7,520 (24.8%) participants were 65 years of age and older. Overall, 52.6% of the participants were male, 47.4% were female, 20.5% were Hispanic or Latino, 79.2% were White, 10.2% were African American, 4.6% were Asian, 0.8% were American Indian or Alaska Native, 0.2% were Native Hawaiian or Pacific Islander, 2.0% were other races, and 2.1% were Multiracial. Demographic characteristics were similar between participants who received SPIKEVAX and those who received placebo.
Solicited Adverse Reactions
Local and systemic adverse reactions and use of antipyretic medication were solicited in an electronic diary for 7 days following each injection (i.e., day of vaccination and the next 6 days) among participants receiving SPIKEVAX (n=15,179) and participants receiving placebo (n=15,159) with at least 1 documented dose. Events that persisted for more than 7 days were followed until resolution. Solicited adverse reactions were reported more frequently among vaccine participants than placebo participants.
The reported number and percentage of the solicited local and systemic adverse reactions by age group and dose are presented in Table 1 and Table 2, respectively.
| SPIKEVAX | Placeboa | |||
|---|---|---|---|---|
| Dose 1
(N=11,406) n (%) | Dose 2
(N=11,000) n (%) | Dose 1
(N=11,402) n (%) | Dose 2
(N=10,929) n (%) |
|
|
Local Adverse Reactions | ||||
|
Pain |
9,908 (86.9) |
9,893 (89.9) |
2,183 (19.1) |
2,048 (18.7) |
|
Pain, Grade 3b |
366 (3.2) |
506 (4.6) |
23 (0.2) |
22 (0.2) |
|
Axillary swelling/tenderness |
1,322 (11.6) |
1,777 (16.2) |
567 (5.0) |
474 (4.3) |
|
Axillary swelling/tenderness, Grade 3b |
37 (0.3) |
47 (0.4) |
13 (0.1) |
12 (0.1) |
|
Swelling (hardness) ≥25 mm |
766 (6.7) |
1,399 (12.7) |
42 (0.4) |
46 (0.4) |
|
Swelling (hardness), Grade 3c |
62 (0.5) |
183 (1.7) |
3 (<0.1) |
5 (<0.1) |
|
Erythema (redness) ≥25 mm |
354 (3.1) |
989 (9.0) |
54 (0.5) |
53 (0.5) |
|
Erythema (redness), Grade 3c |
34 (0.3) |
210 (1.9) |
11 (<0.1) |
12 (0.1) |
|
Systemic Adverse Reactions | ||||
|
Fatigue |
4,385 (38.5) |
7,453 (67.8) |
3,281 (28.8) |
2,701 (24.7) |
|
Fatigue, Grade 3d |
121 (1.1) |
1,178 (10.7) |
83 (0.7) |
88 (0.8) |
|
Fatigue, Grade 4e |
1 (<0.1) |
0 (0) |
0 (0) |
0 (0) |
|
Headache |
4,028 (35.3) |
6,929 (63.0) |
3,303 (29.0) |
2,775 (25.4) |
|
Headache, Grade 3f |
220 (1.9) |
559 (5.1) |
163 (1.4) |
132 (1.2) |
|
Myalgia |
2,700 (23.7) |
6,789 (61.7) |
1,625 (14.3) |
1,425 (13.0) |
|
Myalgia, Grade 3d |
74 (0.6) |
1,116 (10.1) |
38 (0.3) |
42 (0.4) |
|
Arthralgia |
1,892 (16.6) |
5,010 (45.6) |
1,327 (11.6) |
1,180 (10.8) |
|
Arthralgia, Grade 3d |
47 (0.4) |
650 (5.9) |
30 (0.3) |
37 (0.3) |
|
Arthralgia, Grade 4e |
1 (<0.1) |
0 (0) |
0 (0) |
0 (0) |
|
Chills |
1,050 (9.2) |
5,357 (48.7) |
730 (6.4) |
662 (6.1) |
|
Chills, Grade 3g |
17 (0.1) |
164 (1.5) |
8 (<0.1) |
15 (0.1) |
|
Nausea/vomiting |
1,068 (9.4) |
2,355 (21.4) |
908 (8.0) |
807 (7.4) |
|
Nausea/vomiting, Grade 3h |
6 (<0.1) |
11 (0.1) |
8 (<0.1) |
8 (<0.1) |
|
Fever |
102 (0.9) |
1,909 (17.4) |
37 (0.3) |
38 (0.3) |
|
Fever, Grade 3i |
10 (<0.1) |
185 (1.7) |
1 (<0.1) |
2 (<0.1) |
|
Fever, Grade 4j |
4 (<0.1) |
12 (0.1) |
4 (<0.1) |
2 (<0.1) |
|
Use of antipyretic or pain medication |
2,656 (23.3) |
6,307 (57.3) |
1,523 (13.4) |
1,254 (11.5) |
* 7 days included day of vaccination and the subsequent 6 days. Events and use of antipyretic or pain medication were collected in the electronic diary (e-diary).
a Placebo was a saline solution.
b Grade 3 pain and axillary swelling/tenderness: Defined as any use of prescription pain reliever; prevents daily activity.
c Grade 3 swelling and erythema: Defined as >100 mm / >10 cm.
d Grade 3 fatigue, myalgia, arthralgia: Defined as significant; prevents daily activity.
e Grade 4 fatigue, arthralgia: Defined as requires emergency room visit or hospitalization.
f Grade 3 headache: Defined as significant; any use of prescription pain reliever or prevents daily activity.
g Grade 3 chills: Defined as prevents daily activity and requires medical intervention.
h Grade 3 nausea/vomiting: Defined as prevents daily activity; requires outpatient intravenous hydration.
i Grade 3 fever: Defined as ≥39.0° – ≤40.0°C / ≥102.1° – ≤104.0°F.
j Grade 4 fever: Defined as >40.0°C / >104.0°F.
| SPIKEVAX | Placeboa | |||
|---|---|---|---|---|
| Dose 1
(N=3,760) n (%) | Dose 2
(N=3,691) n (%) | Dose 1
(N=3,749) n (%) | Dose 2
(N=3,649) n (%) |
|
|
Local Adverse Reactions | ||||
|
Pain |
2,780 (73.9) |
3,071 (83.2) |
482 (12.9) |
438 (12.0) |
|
Pain, Grade 3b |
50 (1.3) |
100 (2.7) |
32 (0.9) |
19 (0.5) |
|
Axillary swelling/tenderness |
231 (6.1) |
315 (8.5) |
155 (4.1) |
97 (2.7) |
|
Axillary swelling/tenderness, Grade 3b |
12 (0.3) |
21 (0.6) |
14 (0.4) |
8 (0.2) |
|
Swelling (hardness) ≥25 mm |
169 (4.5) |
408 (11.1) |
23 (0.6) |
14 (0.4) |
|
Swelling (hardness), Grade 3c |
20 (0.5) |
72 (2.0) |
3 (<0.1) |
7 (0.2) |
|
Erythema (redness) ≥25 mm |
91 (2.4) |
285 (7.7) |
23 (0.6) |
15 (0.4) |
|
Erythema (redness), Grade 3c |
8 (0.2) |
77 (2.1) |
2 (<0.1) |
3 (<0.1) |
|
Systemic Adverse Reactions | ||||
|
Fatigue |
1,251 (33.3) |
2,154 (58.4) |
852 (22.7) |
717 (19.6) |
|
Fatigue, Grade 3d |
30 (0.8) |
255 (6.9) |
22 (0.6) |
20 (0.5) |
|
Headache |
922 (24.5) |
1,708 (46.3) |
723 (19.3) |
652 (17.9) |
|
Headache, Grade 3e |
53 (1.4) |
107 (2.9) |
34 (0.9) |
33 (0.9) |
|
Myalgia |
742 (19.7) |
1,740 (47.2) |
444 (11.9) |
399 (10.9) |
|
Myalgia, Grade 3d |
17 (0.5) |
205 (5.6) |
9 (0.2) |
10 (0.3) |
|
Arthralgia |
618 (16.4) |
1,293 (35.1) |
457 (12.2) |
399 (10.9) |
|
Arthralgia, Grade 3d |
13 (0.3) |
125 (3.4) |
8 (0.2) |
7 (0.2) |
|
Chills |
201 (5.3) |
1,143 (31.0) |
148 (4.0) |
151 (4.1) |
|
Chills, Grade 3f |
7 (0.2) |
27 (0.7) |
6 (0.2) |
2 (<0.1) |
|
Nausea/vomiting |
194 (5.2) |
439 (11.9) |
167 (4.5) |
134 (3.7) |
|
Nausea/vomiting, Grade 3g |
4 (0.1) |
10 (0.3) |
5 (0.1) |
3 (<0.1) |
|
Nausea/vomiting, Grade 4h |
0 (0) |
1 (<0.1) |
0 (0) |
0 (0) |
|
Fever |
10 (0.3) |
367 (9.9) |
7 (0.2) |
5 (0.1) |
|
Fever, Grade 3i |
1 (<0.1) |
18 (0.5) |
1 (<0.1) |
0 (0) |
|
Fever, Grade 4j |
0 (0) |
1 (<0.1) |
2 (<0.1) |
1 (<0.1) |
|
Use of antipyretic or pain medication |
673 (17.9) |
1,548 (41.9) |
477 (12.7) |
331 (9.1) |
* 7 days included day of vaccination and the subsequent 6 days. Events and use of antipyretic or pain medication were collected in the electronic diary (e-diary).
a Placebo was a saline solution.
b Grade 3 pain and axillary swelling/tenderness: Defined as any use of prescription pain reliever; prevents daily activity.
c Grade 3 swelling and erythema: Defined as >100 mm / >10 cm.
d Grade 3 fatigue, myalgia, arthralgia: Defined as significant; prevents daily activity.
e Grade 3 headache: Defined as significant; any use of prescription pain reliever or prevents daily activity.
f Grade 3 chills: Defined as prevents daily activity and requires medical intervention.
g Grade 3 nausea/vomiting: Defined as prevents daily activity; requires outpatient intravenous hydration.
h Grade 4 nausea/vomiting: Defined as requires emergency room visit or hospitalization for hypotensive shock.
i Grade 3 fever: Defined as ≥39.0° – ≤40.0°C / ≥102.1° – ≤104.0°F.
j Grade 4 fever: Defined as >40.0°C / >104.0°F.
Solicited local and systemic adverse reactions reported following administration of SPIKEVAX had a median duration of 1 to 3 days.
Grade 3 solicited local adverse reactions were more frequently reported after Dose 2 than after Dose 1. Solicited systemic adverse reactions were more frequently reported by vaccine recipients after Dose 2 than after Dose 1.
In Study 1, 2.3% of participants (vaccine=347, placebo=337) had evidence of prior SARS-CoV-2 infection at baseline (immunologic or virologic evidence of prior SARS-CoV-2 infection [defined as positive RT-PCR test and/or positive Elecsys immunoassay result at Day 1]). Overall, among the 347 vaccine participants, there were no notable differences in reactogenicity compared to the 14,750 vaccine participants who had no evidence of prior SARS-CoV-2 infection at baseline (negative RT-PCR test and negative Elecsys immunoassay result at Day 1).
Unsolicited Adverse Events
Participants were monitored for unsolicited adverse events for 28 days following each dose. Serious adverse events and medically attended adverse events will be recorded for the entire study duration (2 years). Among the 30,346 participants who had received at least 1 dose of vaccine (N=15,184) or placebo (N=15,162), unsolicited adverse events that occurred within 28 days following any vaccination were reported by 31.3% of participants (n=4,752) who received SPIKEVAX and 28.6% of participants (n=4,338) who received placebo.
During the 28-day follow-up period following any dose, lymphadenopathy-related events were reported by 1.7% of vaccine recipients and 0.8% of placebo recipients. These events included lymphadenopathy, lymphadenitis, lymph node pain, vaccination-site lymphadenopathy, injection-site lymphadenopathy, and axillary mass. This imbalance is consistent with the imbalance observed for solicited axillary swelling/tenderness at the injected arm.
During the 7-day follow-up period of any vaccination, hypersensitivity events of injection site rash or injection site urticaria, likely related to vaccination, were reported by 6 participants in the SPIKEVAX group and none in the placebo group. Delayed injection site reactions that began >7 days after vaccination were reported in 1.4% of vaccine recipients and 0.7% of placebo recipients. Delayed injection site reactions included pain, erythema, and swelling and are likely related to vaccination.
In the blinded portion of the study, there were 8 reports of facial paralysis (including Bell’s palsy) in the SPIKEVAX group, and 3 in the placebo group. In the 28-day follow-up period there were two cases of facial paralysis in the SPIKEVAX group, which occurred on 8 and 22 days, respectively, after vaccination, and one in the placebo group, which occurred 17 days after vaccination. Currently available information on facial paralysis is insufficient to determine a causal relationship with the vaccine.
In the blinded portion of the study, there were 50 reports of herpes zoster in the SPIKEVAX group, and 23 in the placebo group. In the 28-day period after any vaccination, there were 22 cases of herpes zoster in the SPIKEVAX group, and 15 in the placebo group. Currently available information on herpes zoster infection is insufficient to determine a causal relationship with the vaccine.
There were no other notable patterns or numerical imbalances between treatment groups for specific categories of adverse events (including other neurologic, neuro-inflammatory, and thrombotic events) that would suggest a causal relationship to SPIKEVAX.
Serious Adverse Events
During the blinded phase of the study, serious adverse events were reported by 1.8% (n=268) of participants who received SPIKEVAX and 1.9% (n=292) of participants who received placebo.
There were three serious adverse events of angioedema/facial swelling in the vaccine group in recipients with a history of injection of dermatological fillers. The onset of swelling was reported 1-2 days after the second dose and was likely related to vaccination.
There were no other notable patterns or imbalances between treatment groups for specific categories of serious adverse events (including neurologic, neuro-inflammatory, and thrombotic events) that would suggest a causal relationship to SPIKEVAX.
6.2 Emergency Use Authorization Experience
The following adverse reactions have been identified during emergency use authorization of SPIKEVAX (Moderna COVID-19 Vaccine). Because these reactions are reported voluntarily, it is not always possible to reliably estimate their frequency or establish a causal relationship to vaccine exposure.
Cardiac Disorders: myocarditis, pericarditis
Immune System Disorders: anaphylaxis, urticaria
Nervous System Disorders: syncope
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Exposure Registry
There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to SPIKEVAX during pregnancy. Women who are vaccinated with SPIKEVAX during pregnancy are encouraged to enroll in the registry by calling 1-866-MODERNA (1-866-663-3762).
Risk Summary
All pregnancies have a risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively. Available data on SPIKEVAX administered to pregnant women are insufficient to inform vaccine-associated risks in pregnancy.
A developmental toxicity study was performed in female rats administered the equivalent of a single human dose of SPIKEVAX twice prior to mating and twice during gestation. The study revealed no evidence of harm to the fetus due to the vaccine (see Animal Data).
Data
Animal Data
In a developmental toxicity study, 0.2 mL of a vaccine formulation containing nucleoside-modified messenger ribonucleic acid (mRNA) (100 mcg) and other ingredients that are included in a 0.5 mL single human dose of SPIKEVAX was administered to female rats by the intramuscular route on four occasions: 28 and 14 days prior to mating, and on gestation days 1 and 13. No vaccine-related fetal malformations or variations and no adverse effect on postnatal development were observed in the study.
8.2 Lactation
Risk Summary
It is not known whether SPIKEVAX is excreted in human milk. Data are not available to assess the effects of SPIKEVAX on the breastfed infant or on milk production/excretion. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for SPIKEVAX and any potential adverse effects on the breastfed infant from SPIKEVAX or from the underlying maternal condition. For preventive vaccines, the underlying maternal condition is susceptibility to disease prevented by the vaccine.
8.4 Pediatric Use
Safety and effectiveness have not been established in persons less than 18 years of age.
8.5 Geriatric Use
Clinical studies of SPIKEVAX included participants 65 years of age and older receiving vaccine or placebo, and their data contribute to the overall assessment of safety and efficacy. In a Phase 3 clinical study, 24.8% (n=7,520) of participants were 65 years of age and older and 4.6% (n=1,398) of participants were 75 years of age and older. Vaccine efficacy in participants 65 years of age and older was 91.5% (95% CI 83.2, 95.7) compared to 93.4% (95% CI 91.1, 95.1) in participants 18 to <65 years of age [see Clinical Studies (14)]. A lower proportion of participants 65 years of age and older reported solicited local and systemic adverse reactions compared to participants 18-64 years of age [see Adverse Reactions (6.1)].
11 DESCRIPTION
SPIKEVAX (COVID-19 Vaccine, mRNA) is a sterile white to off-white suspension for intramuscular injection. Each 0.5 mL dose of SPIKEVAX contains 100 mcg of nucleoside-modified messenger RNA (mRNA) encoding the pre-fusion stabilized Spike glycoprotein (S) of SARS-CoV-2 virus.
Each 0.5 mL dose of SPIKEVAX also contains the following ingredients: a total lipid content of 1.93 mg (SM-102, polyethylene glycol [PEG] 2000 dimyristoyl glycerol [DMG], cholesterol, and 1,2-distearoyl-sn-glycero-3-phosphocholine [DSPC]), 0.31 mg tromethamine, 1.18 mg tromethamine hydrochloride, 0.043 mg acetic acid, 0.20 mg sodium acetate trihydrate, and 43.5 mg sucrose.
SPIKEVAX does not contain a preservative.
The vial stoppers are not made with natural rubber latex.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The nucleoside-modified mRNA in SPIKEVAX is encapsulated in lipid particles, which enable delivery of the nucleoside-modified mRNA into host cells to allow expression of the SARS-CoV-2 S antigen. The vaccine elicits an immune response to the S antigen, which protects against COVID-19.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
SPIKEVAX has not been evaluated for carcinogenic, mutagenic potential, or impairment of male fertility in animals. A developmental toxicity study was conducted in female rats that received a vaccine formulation containing nucleoside-modified messenger ribonucleic acid (mRNA) (100 mcg) and other ingredients included in a single human dose of SPIKEVAX. No impact on female fertility was reported (see Use in Specific Populations [8.1]).
14 CLINICAL STUDIES
Study 1 is an ongoing Phase 3 randomized, placebo-controlled, observer-blind clinical trial to evaluate the efficacy, safety, and immunogenicity of SPIKEVAX in participants 18 years of age and older in the United States. Randomization was stratified by age and health risk: 18 to <65 years of age without comorbidities (not at risk for progression to severe COVID-19), 18 to <65 years of age with comorbidities (at risk for progression to severe COVID-19), and 65 years of age and older with or without comorbidities. Participants who were immunocompromised and those with a known history of SARS-CoV-2 infection were excluded from the study. Participants with no known history of SARS-CoV-2 infection but with positive laboratory results indicative of infection at study entry were included. The study allowed for the inclusion of participants with stable pre-existing medical conditions, defined as disease not requiring significant change in therapy or hospitalization for worsening disease during the 3 months before enrollment, as well as participants with stable human immunodeficiency virus (HIV) infection. A total of 30,415 participants were randomized equally to receive 2 doses of SPIKEVAX or saline placebo 1 month apart. Participants will be followed for efficacy and safety until 2 years after the second dose.
The primary efficacy analysis population (referred to as the Per-Protocol Set) included 28,451 participants who received two doses (at 0 and 1 month) of either SPIKEVAX (n=14,287) or placebo (n=14,164), and had a negative baseline SARS-CoV-2 status. In the Per-Protocol Set, 47.5% of participants were female, 19.7% were Hispanic or Latino; 79.7% were White, 9.7% were African American, 4.7% were Asian, and 2.0% other races. The median age of participants was 53 years (range 18-95) and 25.4% of participants were 65 years of age and older. Of the study participants in the Per-Protocol Set, 22.8% were at increased risk of severe COVID-19 due to at least one pre-existing medical condition (chronic lung disease, significant cardiac disease, severe obesity, diabetes, liver disease, or HIV infection) regardless of age. There were no notable differences in demographics or pre-existing medical conditions between participants who received SPIKEVAX and those who received placebo.
The population for the vaccine efficacy analysis included participants 18 years of age and older who were enrolled from July 27, 2020, and followed for the development of COVID-19 through the data cutoff of March 26, 2021, or the Participant Decision Visit for treatment unblinding, whichever was earlier. The median length of follow-up for participants in the blinded placebo-controlled phase of the study was 4 months following Dose 2.
Efficacy Against COVID-19
COVID-19 was defined based on the following criteria: The participant must have experienced at least two of the following systemic symptoms: fever (≥38ºC / ≥100.4ºF), chills, myalgia, headache, sore throat, new olfactory and taste disorder(s); or the participant must have experienced at least one of the following respiratory signs/symptoms: cough, shortness of breath or difficulty breathing, or clinical or radiographical evidence of pneumonia; and the participant must have at least one NP swab, nasal swab, or saliva sample (or respiratory sample, if hospitalized) positive for SARS-CoV-2 by RT-PCR. COVID-19 cases were adjudicated by a Clinical Adjudication Committee.
There were 55 COVID-19 cases in the SPIKEVAX group and 744 cases in the placebo group, with a vaccine efficacy of 93.2% (95% confidence interval of 91.0% to 94.8%) (Table 3).
SARS-CoV-2 identified in the majority of COVID-19 cases in this study were sequenced to be the B.1.2 variant. Additional SARS-CoV-2 variants identified in this study included B.1.427/B.1.429 (Epsilon), P.1 (Gamma), and P.2 (Zeta). Representation of identified variants among cases in the vaccine versus placebo recipients did not suggest decreased vaccine effectiveness against these variants.
|
Age Subgroup (Years) |
SPIKEVAX |
Placebo |
% Vaccine Efficacy (95% CI)† |
||||
|
Participants(N) |
COVID-19 Cases (n) |
Incidence Rate of COVID-19 per 1,000 Person-Years |
Participants (N) |
COVID-19 Cases (n) |
Incidence Rate of COVID-19 per 1,000 Person-Years |
||
|
All participants |
14,287 |
55 |
9.6 |
14,164 |
744 |
136.6 |
93.2 (91.0, 94.8) |
|
18 to <65 |
10,661 |
46 |
10.7 |
10,569 |
644 |
159.0 |
93.4 (91.1, 95.1) |
|
≥65 |
3,626 |
9 |
6.2 |
3,595 |
100 |
71.7 |
91.5 (83.2, 95.7) |
* COVID-19: symptomatic COVID-19 requiring positive RT-PCR result and at least two systemic symptoms (fever [≥38ºC / ≥100.4ºF], chills, myalgia, headache, sore throat, new olfactory and taste disorder[s]) or one respiratory symptom (cough, shortness of breath or difficulty breathing, or clinical or radiographical evidence of pneumonia). Cases starting 14 days after Dose 2.
† VE and 95% CI from the stratified Cox proportional hazard model.
Severe COVID-19 was defined based on confirmed COVID-19 as per the primary efficacy endpoint case definition, plus any of the following: Clinical signs indicative of severe systemic illness, respiratory rate ≥30 per minute, heart rate ≥125 beats per minute, SpO2 ≤93% on room air at sea level or PaO2/FIO2 <300 mm Hg; or respiratory failure or ARDS (defined as needing high-flow oxygen, non-invasive or mechanical ventilation, or ECMO), evidence of shock (systolic blood pressure <90 mmHg, diastolic BP <60 mmHg or requiring vasopressors); or significant acute renal, hepatic, or neurologic dysfunction; or admission to an intensive care unit or death.
Among all participants in the Per-Protocol Set analysis, which included COVID-19 cases confirmed by an adjudication committee, 2 cases of severe COVID-19 were reported in the SPIKEVAX group compared with 106 cases reported in the placebo group, with a vaccine efficacy of 98.2% (95% confidence interval of 92.8% to 99.6%) (Table 4).
|
SPIKEVAX |
Placebo |
% Vaccine Efficacy (95% CI)† |
||||
|
Participants (N) |
Severe COVID-19 Cases (n) |
Incidence Rate of COVID-19 per 1,000 Person-Years |
Participants (N) |
Severe COVID-19 Cases (n) |
Incidence Rate of COVID-19 per 1,000 Person-Years |
|
|
14,287 |
2 |
0.3 |
14,164 |
106 |
19.1 |
98.2 (92.8, 99.6) |
* Severe COVID-19: symptomatic COVID-19 requiring positive RT-PCR result and at least two systemic symptoms or one respiratory symptom, plus any of the following: Clinical signs indicative of severe systemic illness, respiratory rate ≥30 per minute, heart rate ≥125 beats per minute, SpO2 ≤93% on room air at sea level or PaO2/FIO2 <300 mm Hg; or respiratory failure or ARDS (defined as needing high-flow oxygen, non-invasive or mechanical ventilation, or ECMO), evidence of shock (systolic blood pressure <90 mmHg, diastolic BP <60 mmHg or requiring vasopressors); or significant acute renal, hepatic, or neurologic dysfunction; or admission to an intensive care unit or death. Cases starting 14 days after Dose 2.
† VE and 95% CI from the stratified Cox proportional hazard model.
In an exploratory analysis, occurrence of asymptomatic SARS-CoV-2 infection was assessed among participants in the Per-Protocol Set (enrolled from July 27, 2020, and followed maximally through March 26, 2021). Asymptomatic SARS-CoV-2 infection was defined as having a positive scheduled serology test based on binding antibody against SARS-CoV-2 nucleocapsid protein as measured by the Roche Elecsys immunoassay (N-serology) and/or a positive RT-PCR test for SARS-CoV-2, in the absence of any reported COVID-19 symptoms included as part of the primary efficacy endpoint case definition (described above) or symptoms included in the secondary COVID-19 endpoint case definition (fever >38°C / ≥100.4°F, chills, cough, shortness of breath or difficulty breathing, fatigue, muscle aches, body aches, headache, new loss of taste or smell, sore throat, congestion or runny nose, nausea, vomiting, or diarrhea) at any time during the study. To assess for asymptomatic infection starting 14 days after Dose 2, all participants had scheduled blood draws for N-serology collected at the 1 month post-Dose 2 visit and the 6 months post-Dose 2 visit (if still blinded to treatment arm), and scheduled N-serology and nasopharyngeal swab for RT-PCR collection at the Participant Decision Visit for treatment unblinding.
In the Per-Protocol Set, 14,287 participants in the SPIKEVAX group and 14,164 participants in the placebo group had N-serology and/or RT-PCR results available from one or more of the pre-specified timepoints listed above. Among these participants, there were 180 cases of asymptomatic SARS-CoV-2 infection in the SPIKEVAX group compared with 399 cases in the placebo group. Limitations of this analysis include the infrequently scheduled assessments for serology and PCR testing, which may not have captured all cases of asymptomatic infections which occurred during the study.
16 HOW SUPPLIED/STORAGE AND HANDLING
SPIKEVAX is supplied in multiple-dose vials as follows:
NDC: 80777-100-99 Carton of 10 multiple-dose vials, each vial containing 5.5 mL
NDC: 80777-100-98 Carton of 10 multiple-dose vials, each vial containing 7.5 mL
During storage, minimize exposure to room light, and avoid exposure to direct sunlight and ultraviolet light.
Frozen Storage
Store frozen between -50°C to -15°C (-58°F to 5°F).
Storage after Thawing
-
Storage at 2°C to 8°C (36°F to 46°F):
- o Vials may be stored refrigerated between 2°C to 8°C (36°F to 46°F) for up to 30 days prior to first use.
- o Vials should be discarded 12 hours after the first puncture.
-
Storage at 8°C to 25°C (46°F to 77°F):
- o Vials may be stored between 8°C to 25°C (46°F to 77°F) for a total of 24 hours.
- o Vials should be discarded 12 hours after the first puncture.
- o Total storage at 8°C to 25°C (46°F to 77°F) must not exceed 24 hours.
Do not refreeze once thawed.
Thawed vials can be handled in room light conditions.
Transportation of Thawed Vials at 2°C to 8°C (36°F to 46°F)
If transport at -50°C to -15°C (-58°F to 5°F) is not feasible, available data support transportation of one or more thawed vials for up to 12 hours at 2°C to 8°C (36°F to 46°F) when shipped using shipping containers which have been qualified to maintain 2°C to 8°C (36°F to 46°F) and under routine road and air transport conditions with shaking and vibration minimized. Once thawed and transported at 2°C to 8°C (36°C to 46°F), vials should not be refrozen and should be stored at 2°C to 8°C (36°F to 46°F) until use.
17 PATIENT COUNSELING INFORMATION
Advise the vaccine recipient or caregiver to read the FDA-approved patient labeling.
Inform the vaccine recipient or caregiver of the potential benefits and risks of vaccination with SPIKEVAX.
Inform the vaccine recipient or caregiver of the importance of completing the two dose vaccination series.
Instruct the vaccine recipient or caregiver to report any adverse events to their healthcare provider or to the Vaccine Adverse Event Reporting System at 1-800-822-7967 and www.vaers.hhs.gov.
There is a pregnancy exposure registry for SPIKEVAX. Encourage individuals who receive SPIKEVAX around the time of conception or while pregnant to enroll in the pregnancy exposure registry. Pregnant individuals can enroll in the pregnancy exposure registry by calling 1-866-MODERNA (1-866-663-3762).
Prior to administering the vaccine, provide the vaccine recipient the Vaccine Information Fact Sheet for Recipients and Caregivers about SPIKEVAX (COVID-19 Vaccine, mRNA) and the Moderna COVID-19 Vaccine to Prevent Coronavirus Disease 2019 (COVID-19) for Use in Individuals 18 Years of Age and Older. The Vaccine Information Fact Sheet for Recipients and Caregivers is available at https://www.modernatx.com/covid19vaccine-eua/eua-fact-sheet-recipients.pdf.
This product’s labeling may have been updated. For the most recent prescribing information, please visit https://fda.report/dailymed/.
Manufactured for:
Moderna US, Inc.
Cambridge, MA 02139
©2022 ModernaTX, Inc. All rights reserved.
SPIKEVAX is a trademark of ModernaTX, Inc.
Patent(s): www.modernatx.com/patents
US Govt. License No. 2256
Revised: 5/2023
Information for Recipients and Caregivers
SPIKEVAX (pronounced SPĪK-văx)
(COVID-19 Vaccine, mRNA)
Please read this information sheet before getting SPIKEVAX. This summary is not intended to take the place of talking with your healthcare provider. If you have questions or would like more information, please talk with your healthcare provider.
What is SPIKEVAX?
SPIKEVAX is a vaccine to help protect you against COVID-19. SPIKEVAX is for people 18 years of age and older. Vaccination with SPIKEVAX may not protect all people who receive the vaccine.
Who should not get SPIKEVAX?
You should not get SPIKEVAX if you:
- had a severe allergic reaction after receiving a previous dose of SPIKEVAX (also called Moderna COVID-19 Vaccine)
- had a severe allergic reaction to any ingredient of this vaccine (see What are the ingredients in SPIKEVAX?)
What should I tell my healthcare provider?
Tell your healthcare provider about all of your medical conditions, including if you:
- have any allergies
- had a severe allergic reaction after receiving a previous dose of any COVID-19 vaccine
- have had myocarditis (inflammation of the heart muscle) or pericarditis (inflammation of the lining outside the heart)
- have a fever
- have a bleeding disorder or are on a blood thinner
- are immunocompromised or are on a medicine that affects your immune system
- are pregnant or plan to become pregnant
- are breastfeeding
- have received any other COVID-19 vaccine
- have ever fainted in association with an injection
How is SPIKEVAX given?
SPIKEVAX is given as an injection into the muscle. The SPIKEVAX vaccination series is 2 doses given 1 month apart. If you receive one dose of SPIKEVAX, you should receive a second dose of SPIKEVAX (also called Moderna COVID-19 Vaccine) 1 month later to complete the vaccination series.
What are the risks of SPIKEVAX?
Severe allergic reactions have occurred in some people who have received SPIKEVAX (also called Moderna COVID-19 Vaccine). There is a very small chance that SPIKEVAX could cause a severe allergic reaction. A severe allergic reaction would usually occur within a few minutes to one hour after getting a dose of SPIKEVAX. For this reason, your healthcare provider may ask you to stay for a short time at the place where you received your vaccine. Signs of a severe allergic reaction can include:
- Difficulty breathing
- Swelling of your face and throat
- A fast heartbeat
- A rash all over your body
- Dizziness and weakness
Myocarditis (inflammation of the heart muscle) and pericarditis (inflammation of the lining outside the heart) have occurred in some people who have received SPIKEVAX (also called Moderna COVID-19 Vaccine), more commonly in males under 40 years of age than among females and older males. In most of these people, symptoms began within a few days following receipt of the second dose of SPIKEVAX. The chance of this happening is very low. You should seek medical attention right away if you have any of the following symptoms after receiving SPIKEVAX:
- Chest pain
- Shortness of breath
- Feelings of having a fast-beating, fluttering, or pounding heart
Side effects that have been reported in clinical trials with SPIKEVAX include:
- Injection site reactions: pain, tenderness and swelling of the lymph nodes in the same arm of the injection, swelling (hardness), and redness
- General side effects: fatigue, headache, muscle pain, joint pain, chills, nausea and vomiting, and fever
Side effects that have been reported outside of clinical trials include:
- Severe allergic reactions
- Urticaria (itchy rash/hives)
- Myocarditis (inflammation of the heart muscle)
- Pericarditis (inflammation of the lining outside the heart)
- Fainting in association with injection of the vaccine
These may not be all of the possible side effects of SPIKEVAX. Ask your healthcare provider about any side effects that concern you. You may report side effects to the Vaccine Adverse Event Reporting System (VAERS) at 1-800-822-7967 or https://vaers.hhs.gov.
What if I am pregnant or breastfeeding?
If you are pregnant or breastfeeding, discuss your options with your healthcare provider.
A pregnancy exposure registry is available. You are encouraged to contact the registry as soon as you become aware of your pregnancy by calling 1-866-MODERNA (1-866-663-3762), or ask your healthcare provider to contact the registry for you.
What are the ingredients in SPIKEVAX?
SPIKEVAX contains the following ingredients: messenger ribonucleic acid (mRNA), lipids (SM-102, polyethylene glycol [PEG] 2000 dimyristoyl glycerol [DMG], cholesterol, and 1,2-distearoyl-sn-glycero-3-phosphocholine [DSPC]), tromethamine, tromethamine hydrochloride, acetic acid, sodium acetate trihydrate, and sucrose.
SPIKEVAX does not contain SARS-CoV-2, the virus that causes COVID-19. SPIKEVAX cannot give you COVID-19.
SPIKEVAX does not contain preservatives.
What else do I need to know?
Keep your vaccination card. When you receive your first dose, you will get a vaccination card to show you when to return for your second dose of SPIKEVAX (also called the Moderna COVID-19 Vaccine). Remember to bring your card when you return.
If you would like more information, talk to your healthcare provider or visit www.spikevax.com or call 1-866-MODERNA (1-866-663-3762).
Manufactured for:
Moderna US, Inc.
Cambridge, MA 02139
©2022 ModernaTX, Inc. All rights reserved.
SPIKEVAX is a trademark of ModernaTX, Inc.
Patent(s): www.modernatx.com/patents
Revised: May 2023
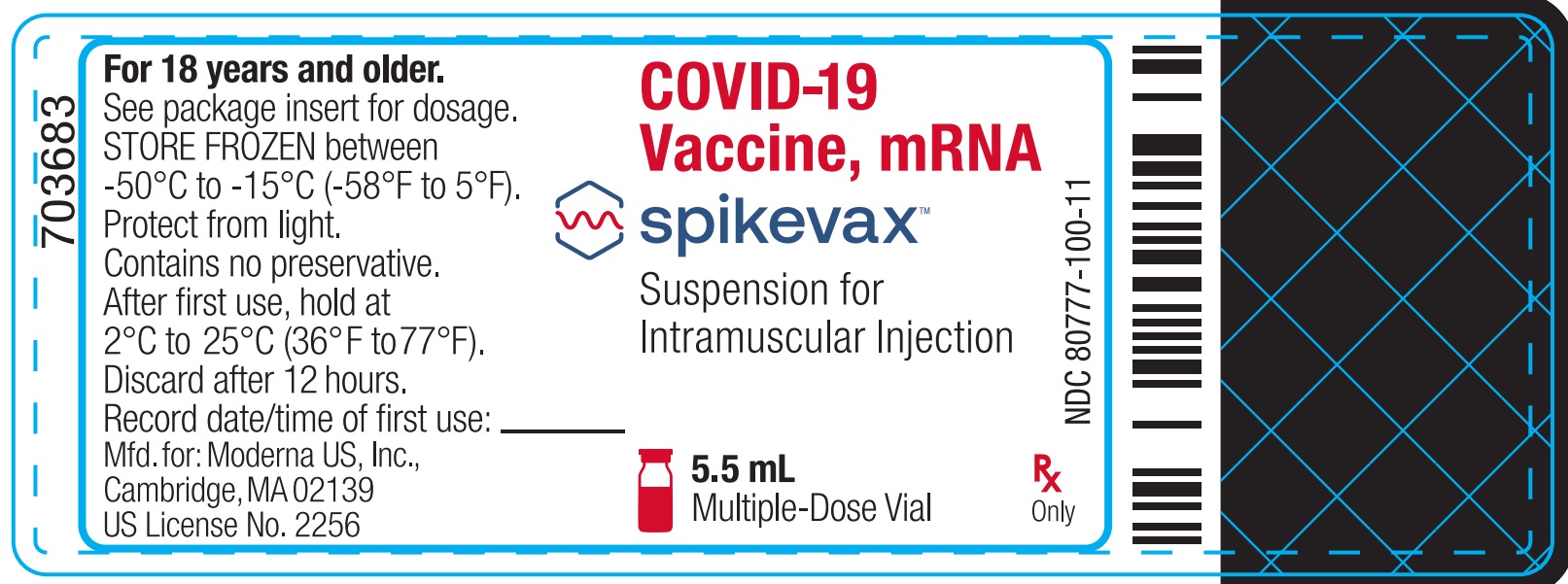
Package/Label Display Panel
NDC: 80777-100-11
COVID-19 Vaccine, mRNA
Spikevax
Suspension for Intramuscular Injection
5.5 mL
Multiple-Dose Vial
Rx Only
Package/Label Display Panel
Moderna
NDC: 80777-100-99
Rx Only
COVID-19 Vaccine, mRNA
Spikevax
Suspension for Intramuscular Injection
For 18 years and older
STORE FROZEN between -50°C to -15°C (-58°F to 5°F).
Protect from light
10 Multiple-Dose Vials (each vial contains 5.5 mL)
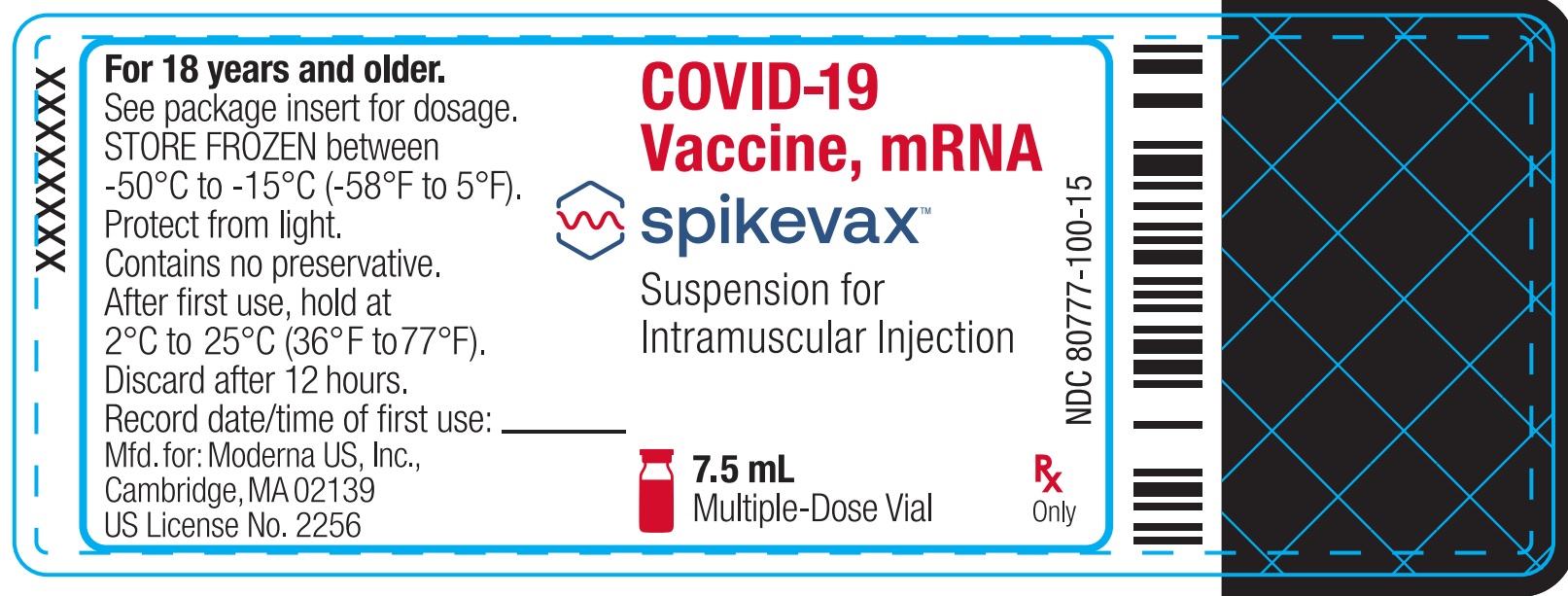
Package/Label Display Panel
NDC: 80777-100-15
COVID-19 Vaccine, mRNA
Spikevax
Suspension for Intramuscular Injection
7.5 mL
Multiple-Dose Vial
Rx Only
Package/Label Display Panel
Moderna
NDC: 80777-100-98
Rx Only
COVID-19 Vaccine, mRNA
Spikevax
Suspension for Intramuscular Injection
For 18 years and older
STORE FROZEN between -50°C to -15°C (-58°F to 5°F).
Protect from light
10 Multiple-Dose Vials (each vial contains 7.5 mL)
| SPIKEVAX
covid-19 vaccine, mrna injection, suspension |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Moderna US, Inc. (117626450) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| ModernaTX, Inc. | 116912313 | ANALYSIS(80777-100) , API MANUFACTURE(80777-100) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Lonza Biologics, Inc. | 093149750 | ANALYSIS(80777-100) , API MANUFACTURE(80777-100) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Catalent Indiana, LLC | 172209277 | MANUFACTURE(80777-100) , LABEL(80777-100) , PACK(80777-100) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Baxter Pharmaceutical Solutions, LLC | 604719430 | MANUFACTURE(80777-100) , ANALYSIS(80777-100) , LABEL(80777-100) , PACK(80777-100) | |
Trademark Results [Spikevax]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 SPIKEVAX 97037862 not registered Live/Pending |
ModernaTx, Inc. 2021-09-21 |
 SPIKEVAX 90247367 not registered Live/Pending |
ModernaTx, Inc. 2020-10-11 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.