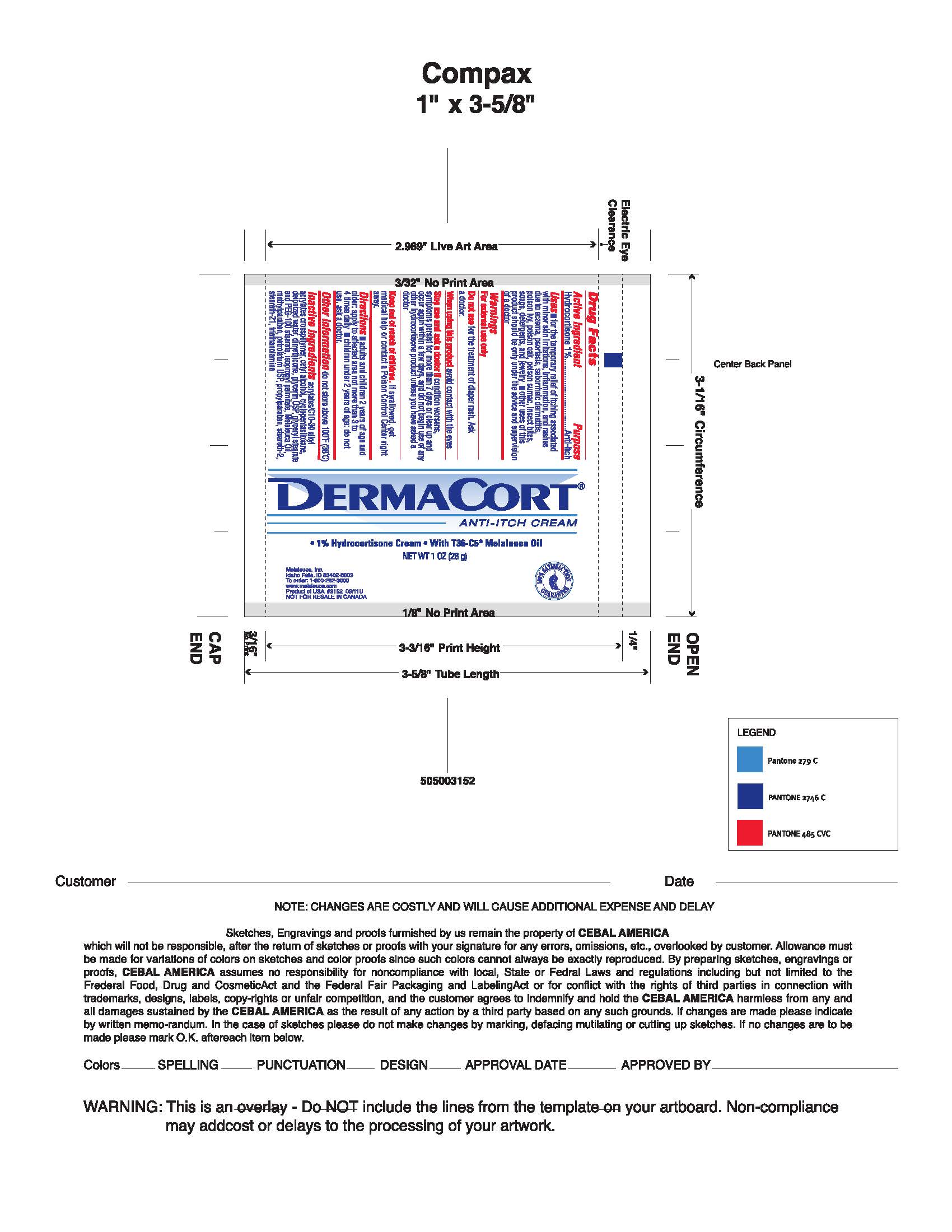
DERMACORT ANTI-ITCH- hydrocortisone cream
DermaCort by
Drug Labeling and Warnings
DermaCort by is a Otc medication manufactured, distributed, or labeled by Melaleuca, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- ACTIVE INGREDIENT
- PURPOSE
-
INDICATIONS & USAGE
Uses
- For the temporary relief of itching associated with minor skin irritations, inflammation and rashes due to eczema, psoriasis, , seborrheic dermatitis, poison ivy, poison oak, poison sumac, insect bites, soaps, detergents, and jewelery
- Other use of this product should be only under the advice and supervision of a doctor.
- WARNINGS
- WHEN USING
- STOP USE
- DO NOT USE
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
- STORAGE AND HANDLING
-
INACTIVE INGREDIENT
Inactive ingredients Acrylates/C10-30 Alkyl Acrylates Crosspolymer, Cetyl Alcohol, Cyclopentasiloxane, Deionized Water, Dimethicone, Glycerin USP, Glyceryl Stearate and PEG-100 Stearate, Isopropyl Palmitate, Melaleuca Oil, Methylparaben, Petrolatum USP, Propylparaben, Serareth-2, Steareth-21, Triethanolamine.
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
DERMACORT ANTI-ITCH
hydrocortisone creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 54473-117 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HYDROCORTISONE (UNII: WI4X0X7BPJ) (HYDROCORTISONE - UNII:WI4X0X7BPJ) HYDROCORTISONE 10 mg in 1 g Inactive Ingredients Ingredient Name Strength POLYOXYL 100 STEARATE (UNII: YD01N1999R) PROPYLPARABEN (UNII: Z8IX2SC1OH) STEARETH-2 (UNII: V56DFE46J5) STEARETH-21 (UNII: 53J3F32P58) TEA TREE OIL (UNII: VIF565UC2G) TROLAMINE (UNII: 9O3K93S3TK) WATER (UNII: 059QF0KO0R) CARBOMER COPOLYMER TYPE B (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: 809Y72KV36) CETYL ALCOHOL (UNII: 936JST6JCN) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) DIMETHICONE (UNII: 92RU3N3Y1O) GLYCERIN (UNII: PDC6A3C0OX) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) ISOPROPYL PALMITATE (UNII: 8CRQ2TH63M) METHYLPARABEN (UNII: A2I8C7HI9T) PETROLATUM (UNII: 4T6H12BN9U) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 54473-117-28 28 g in 1 TUBE; Type 0: Not a Combination Product 01/01/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 08/02/2000 Labeler - Melaleuca, Inc. (139760102) Registrant - Melaleuca, Inc. (139760102) Establishment Name Address ID/FEI Business Operations Melaleuca, Inc. 079711683 manufacture(54473-117)
Trademark Results [DermaCort]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 DERMACORT 78263550 2834681 Live/Registered |
Melaleuca, Inc. 2003-06-17 |
 DERMACORT 76352442 not registered Dead/Abandoned |
Melaleuca, Inc. 2001-12-26 |
 DERMACORT 74404201 1835132 Dead/Cancelled |
Solvay Pharmaceuticals, Inc. 1993-06-21 |
 DERMACORT 72347320 0909980 Dead/Expired |
ROWELL LABORATORIES, INC. 1969-12-29 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.