Aceast Clear concealer sunscreen liquid foundation SPF50 PA by Guangzhou Meixi Biotechnology Co., Ltd. 84167-005 Completed
Aceast Clear concealer sunscreen liquid foundation SPF50 PA by
Drug Labeling and Warnings
Aceast Clear concealer sunscreen liquid foundation SPF50 PA by is a Otc medication manufactured, distributed, or labeled by Guangzhou Meixi Biotechnology Co., Ltd.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
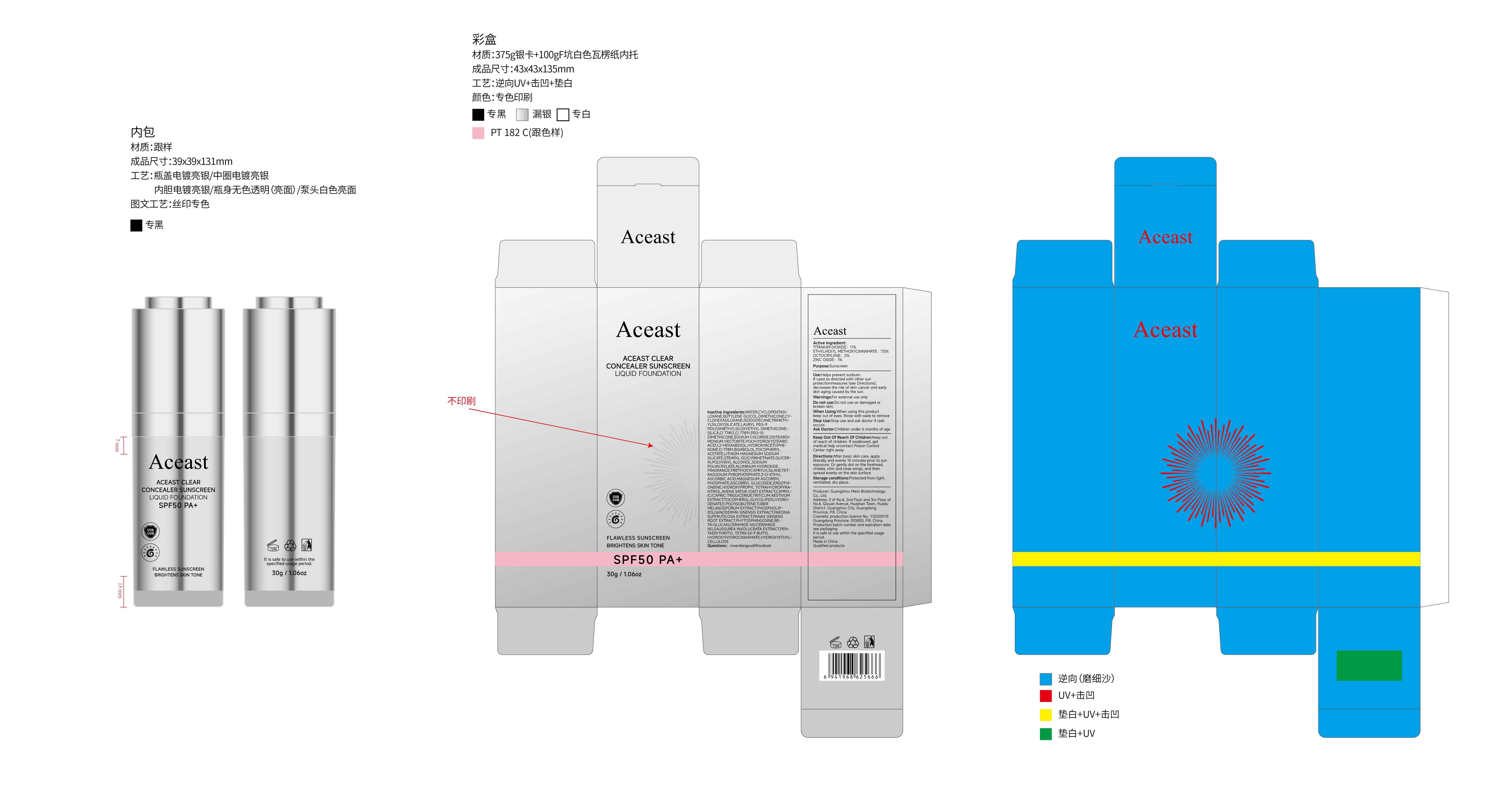
ACEAST CLEAR CONCEALER SUNSCREEN LIQUID FOUNDATION SPF50 PA- clear concealer sunscreen liquid foundation spf50 pa liquid
Guangzhou Meixi Biotechnology Co., Ltd.
----------
84167-005 Completed
Active Ingredient
TITANIUM DIOXIDE 11%
ETHYLHEXYL METHOXYCINNAMATE 7.5%
OCTOCRYLENE 2%
ZINC OXIDE 1%
Use
Helps prevent sunbum.
lf used as directed with other sun protectionmeasures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun.
Keep Oot Of Reach Of Children
Keep out of reach of children. lf swallowed, get medical help orcontact Poison Control Center right away
Directions
After basic skin care, apply liberally and evenly 15 minutes prior to sun exposure. Or gently dot on the forehead, cheeks, chin and nose wings, and then spread evenly on the skin surface
Inactive ingredients
WATER,CYCLOPENTASILOXANE,BUTYLENE GLYCOL,DIMETHICONE
,CYCLOHEXASILOXANE,ISODODECANE,TRIMETHYLSILOXYSILICATE,LAURYL PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE,SILICA,CI 77492,CI 77891,SODIUM CHLORIDE,DISTEARDIMONIUM HECTORITE,POLYHYDROXYSTEARIC ACID,1,2-HEXANEDIOL,HYDROXYACETOPHENONE,CI 77491,BISABOLOL,TOCOPHERYL ACETATE,STEARYL GLYCYRRHETINATE,GLYCERIN,POLYVINYL ALCOHOL,SODIUM POLYACRYLATE,ALUMINUM HYDROXIDE,FRAGRANCE,TRIETHOXYCAPRYLYLSILANE,TETRASODIUM PYROPHOSPHATE,AVENA SATIVA (OAT) EXTRACT,MAGNESIUM ASCORBYL PHOSPHATE,3-O-ETHYL ASCORBIC ACID,ASCORBYL GLUCOSIDE,ERGOTHIONEINE,HYDROXYPROPYL TETRAHYDROPYRANTRIOL,CAPRYLIC/CAPRIC TRIGLYCERIDE,TRITICUM AESTIVUM EXTRACT,TOCOPHEROL,GLYCOLIPIDS,HYDROGENATED POLYISOBUTENE,PHOSPHOLIPIDS,TUBER MELANOSPORUM EXTRACT,GANODERMA SINENSIS EXTRACT,PAEONIA SUFFRUTICOSA EXTRACT,PANAX GINSENG ROOT EXTRACT,PHYTOSPHINGOSINE,BETA-GLUCAN,SAUSSUREA INVOLUCRATA EXTRACT,CERAMIDE NS/CERAMIDE NG,PENTAERYTHRITYL TETRA-DI-T-BUTYL HYDROXYHYDROCINNAMATE,HYDROXYETHYLCELLULOSE
| ACEAST CLEAR CONCEALER SUNSCREEN LIQUID FOUNDATION SPF50 PA
clear concealer sunscreen liquid foundation spf50 pa liquid |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Guangzhou Meixi Biotechnology Co., Ltd. (625724571) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Guangzhou Meixi Biotechnology Co., Ltd. | 625724571 | manufacture(84167-005) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.