ALFERON- interferon alfa-n3 injection
Alferon by
Drug Labeling and Warnings
Alferon by is a Prescription medication manufactured, distributed, or labeled by AIM ImmunoTech Inc, AIM ImmunoTech Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Storage
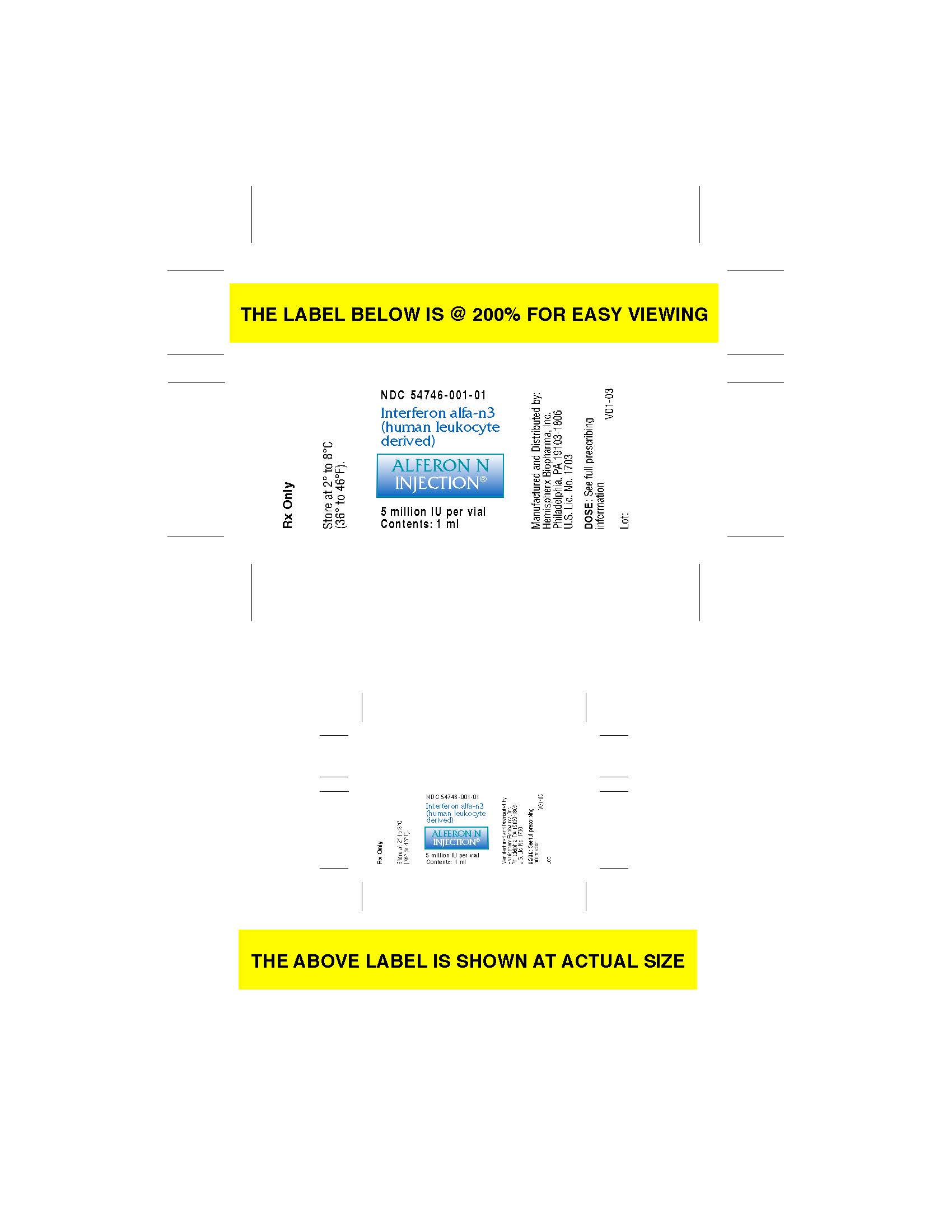
- carton label
- vial label
-
INGREDIENTS AND APPEARANCE
ALFERON
interferon alfa-n3 injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 54746-001 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength INTERFERON ALFA-N3 (UNII: 47BPR3V3MP) (INTERFERON ALFA-N3 - UNII:47BPR3V3MP) INTERFERON ALFA-N3 5000000 [arb'U] in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 54746-001-01 00 mL in 1 VIAL, GLASS; Type 0: Not a Combination Product 10/10/1989 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103158 10/10/1989 Labeler - AIM ImmunoTech Inc (058608076) Registrant - AIM ImmunoTech Inc (119520661) Establishment Name Address ID/FEI Business Operations AIM ImmunoTech Inc. 119520661 api manufacture(54746-001)
Trademark Results [Alferon]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ALFERON 73608837 1427170 Live/Registered |
INTERFERON SCIENCES, INC. 1986-07-10 |
 ALFERON 71456790 0400889 Dead/Cancelled |
DRIVER-HARRIS COMPANY 1942-11-13 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.