DUODOTE- atropine and pralidoxime chloride kit
DuoDote by
Drug Labeling and Warnings
DuoDote by is a Prescription medication manufactured, distributed, or labeled by Meridian Medical Technologies® LLC, Meridian Medical Technologies LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use DUODOTE® safely and effectively. See full prescribing information for DUODOTE.
DUODOTE (atropine and pralidoxime chloride injection), for intramuscular use
Initial U.S. Approval: 2006INDICATIONS AND USAGE
DuoDote, a combination of atropine, a cholinergic muscarinic antagonist, and pralidoxime chloride, a cholinesterase reactivator, is indicated for the treatment of poisoning by organophosphorus nerve agents as well as organophosphorus insecticides in adults and pediatric patients weighing more than 41 kg (90 pounds). (1)
DOSAGE AND ADMINISTRATION
- DuoDote is intended as an initial treatment as soon as symptoms appear; definitive medical care should be sought immediately. (2.1)
- Dosage for Mild Symptoms: If the patient experiences two or more mild symptoms, administer one injection intramuscularly into the mid-lateral thigh. If, at any time after the first dose, the patient develops any of the severe symptoms, administer two additional injections intramuscularly in rapid succession. (2.2)
- Dosage for Severe Symptoms: If a patient has any of the severe symptoms, immediately administer three injections intramuscularly into the patient's mid-lateral thigh in rapid succession. (2.2)
DOSAGE FORMS AND STRENGTHS
Each single-dose DuoDote autoinjector contains atropine (2.1 mg/0.7 mL) plus pralidoxime chloride (600 mg/2 mL). (3)
CONTRAINDICATIONS
None. (4)
WARNINGS AND PRECAUTIONS
- Cardiovascular (CV) Risks: Tachycardia, palpitations, premature ventricular contractions, flutter, fibrillation, etc. Use caution in patients with known CV disease or conduction problems. (5.1)
- Heat Injury: May inhibit sweating and lead to hyperthermia; avoid excessive exercising and heat exposure. (5.2)
- Acute Glaucoma: May precipitate in susceptible individuals. (5.3)
- Urinary Retention: Administer with caution in patient with bladder outflow obstruction. (5.4)
- Pyloric Stenosis: May convert into complete obstruction. (5.5)
- Exacerbation of Chronic Lung Disease: Atropine may cause inspiration of bronchial secretions and formation of dangerous viscid plugs in individuals with chronic lung disease; monitor respiratory status. (5.6)
ADVERSE REACTIONS
Common adverse reactions of atropine include dryness of mouth, blurred vision, dry eyes, photophobia, confusion, headache, and dizziness among others. (6.1) The common adverse reactions of pralidoxime chloride include changes in vision, dizziness, headache, drowsiness, nausea, tachycardia, increased blood pressure, muscular weakness, dry mouth, emesis, rash, dry skin, hyperventilation, decreased renal function, excitement, manic behavior, and transient elevation of liver enzymes and creatine phosphokinase. (6.2)
To report SUSPECTED ADVERSE REACTIONS, contact Pfizer Inc. at 1-800-438-1985 or www.meridianmeds.com or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- Succinylcholine and Mivacurium: Accelerated reversal of neuromuscular blocking effects may occur; monitor with concomitant administration. (7.1)
USE IN SPECIFIC POPULATIONS
- Geriatric patients may be more susceptible to the effects of atropine. (8.5)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 10/2017
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Information
2.2 Dosage Information
2.3 Administration Instructions
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Cardiovascular Risks
5.2 Heat Injury
5.3 Acute Glaucoma
5.4 Urinary Retention
5.5 Pyloric Stenosis
5.6 Exacerbation of Chronic Lung Disease
6 ADVERSE REACTIONS
6.1 Atropine
6.2 Pralidoxime Chloride
6.3 Injection Site
6.4 Inadvertent Injection
7 DRUG INTERACTIONS
7.1 Succinylcholine and Mivacurium
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
10 OVERDOSAGE
10.1 Symptoms
10.2 Treatment
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Information
- Three (3) DuoDote autoinjectors should be available for use in each patient (including healthcare providers) at risk for organophosphorus poisoning; one (1) for mild symptoms plus two (2) more for severe symptoms [see Dosage and Administration (2.2)]. Note that individuals may not have all symptoms included under the mild or severe symptom category.
- Only administer DuoDote to patients experiencing symptoms of organophosphorus poisoning in a situation where exposure is known or suspected. The DuoDote autoinjector is intended as an initial treatment of the symptoms of organophosphorus nerve agent or insecticide poisonings as soon as symptoms appear; definitive medical care should be sought immediately.
- The DuoDote autoinjector should be administered by healthcare providers who have had adequate training in the recognition and treatment of nerve agent or insecticide intoxication.
- Close supervision of all treated patients is indicated for at least 48 to 72 hours.
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit [see Dosage Forms and Strengths (3)].
2.2 Dosage Information
Dosage for Mild Symptoms in Adults and Pediatric Patients Weighing More Than 41 kg (90 Pounds)
First Dose: If the patient experiences two or more mild symptoms of nerve agent or insecticide exposure listed in Table 1, administer one (1) DuoDote injection intramuscularly into the mid-lateral thigh.
Additional Doses: If, at any time after the first dose, the patient develops any of the severe symptoms listed in Table 1, administer two (2) additional DuoDote injections intramuscularly in rapid succession.
Wait 10 to15 minutes for DuoDote to take effect. If, after 10 to15 minutes, the patient does not develop any of the severe symptoms listed in Table 1, no additional DuoDote injections are recommended.
Dosage for Severe Symptoms in Adults and Pediatric Patients Weighing More Than 41 kg (90 Pounds)
If a patient has any of the severe symptoms listed in Table 1, immediately administer three (3) DuoDote injections intramuscularly into the patient's mid-lateral thigh in rapid succession.
Table 1. Common Symptoms of Organophosphorus Exposure Mild Symptoms Severe Symptoms - Blurred vision, miosis
- Excessive, unexplained teary eyes
- Excessive, unexplained runny nose
- Increased salivation such as sudden drooling
- Chest tightness or difficulty breathing
- Tremors throughout the body or muscular twitching
- Nausea and/or vomiting
- Unexplained wheezing, coughing or increased airway secretions
- Acute onset of stomach cramps
- Tachycardia or bradycardia
- Strange or confused behavior
- Severe difficulty breathing or copious secretions from lungs/airway
- Severe muscular twitching and general weakness
- Involuntary urination and defecation
- Convulsions
- Unconsciousness
2.3 Administration Instructions
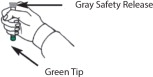
*Do Not Remove Gray Safety Release until ready to use.
*Never touch the Green Tip (Needle End)!
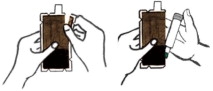
- Tear open the plastic pouch at any of the notches. Remove the DuoDote autoinjector from the pouch.
- Place the DuoDote autoinjector in your dominant hand. (If you are right-handed, your right hand is dominant.) Firmly grasp the center of the DuoDote autoinjector with the Green Tip (needle end) pointing down.
- With your other hand, pull off the Gray Safety Release. DuoDote is now ready to be administered.
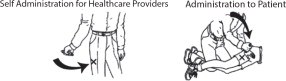
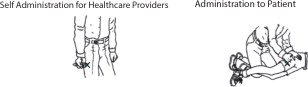
- The injection site is the mid-lateral thigh area. The DuoDote autoinjector can inject through clothing. However, make sure pockets at the injection site are empty. People who may not have a lot of fat at the injection site should also be injected in the mid-lateral thigh, but before giving the injection, bunch up the thigh to provide a thicker area for injection.
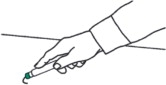
- Firmly push the Green Tip straight down (a 90° angle) against the mid-lateral thigh. Continue to firmly push until you feel the DuoDote autoinjector trigger. After the autoinjector triggers, hold the DuoDote autoinjector firmly in place against the injection site for approximately 10 seconds.
- Remove the DuoDote autoinjector from the thigh and look at Green Tip. If the needle is visible, the drug has been administered. If the needle is not visible, check to be sure the Gray Safety Release has been removed, and then repeat above steps beginning with Step 4, but push harder in Step 5.
- After the drug has been administered, push the needle against a hard surface to bend the needle back against the DuoDote autoinjector.
- Put the used DuoDote autoinjector back into the plastic pouch, if available. Leave used DuoDote autoinjector(s) with the patient to allow other medical personnel to see the number of DuoDote autoinjector(s) administered.
- Immediately move yourself and the patient away from the contaminated area and seek definitive medical care for the patient.
-
3 DOSAGE FORMS AND STRENGTHS
Each single-dose DuoDote autoinjector contains the following in two separate chambers:
- front chamber (visible): a clear, colorless to yellow, sterile solution of atropine (2.1 mg/0.7 mL)
- back chamber (not visible): a clear, colorless to yellow, sterile solution of pralidoxime chloride (600 mg/2 mL) equivalent to pralidoxime (476.6 mg/2 mL)
When activated, DuoDote sequentially administers both drugs intramuscularly through a single needle in one injection.
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Cardiovascular Risks
Cardiovascular adverse reactions reported in the literature for atropine include, but are not limited to, sinus tachycardia, palpitations, premature ventricular contractions, atrial flutter, atrial fibrillation, ventricular flutter, ventricular fibrillation, cardiac syncope, asystole, and myocardial infarction. In patients with a recent myocardial infarction and/or severe coronary artery disease, there is a possibility that atropine-induced tachycardia may cause ischemia, extend or initiate myocardial infarcts, and stimulate ventricular ectopy and fibrillation. DuoDote should be used with caution in patients with known cardiovascular disease or cardiac conduction problems.
5.2 Heat Injury
Atropine may inhibit sweating which, in a warm environment or with excessive exercise, can lead to hyperthermia and heat injury. To the extent feasible, avoid excessive exercise and heat exposure [see Overdosage (10.2)].
5.3 Acute Glaucoma
Atropine should be administered with caution in patients at risk for acute glaucoma.
5.4 Urinary Retention
Atropine should be administered with caution in patients with clinically significant bladder outflow obstruction because of the risk of urinary retention.
-
6 ADVERSE REACTIONS
The following serious adverse reactions are described elsewhere in the labeling:
- Cardiovascular Risks [see Warnings and Precautions (5.1)]
- Heat Injury [see Warnings and Precautions (5.2)]
- Acute Glaucoma [see Warnings and Precautions (5.3)]
- Urinary Retention [see Warnings and Precautions (5.4)]
- Pyloric Stenosis [see Warnings and Precautions (5.5)]
- Exacerbation of Chronic Lung Disease [see Warnings and Precautions (5.6)]
The following adverse reactions associated with the use of atropine and pralidoxime chloride were identified in the literature. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
6.1 Atropine
Because DuoDote contains pralidoxime chloride, which may potentiate the effect of atropine, signs of atropinization may occur earlier than might be expected when atropine is used alone.
Common adverse reactions of atropine can be attributed to its antimuscarinic action. These include dryness of the mouth, blurred vision, dry eyes, photophobia, confusion, headache, dizziness, tachycardia, palpitations, flushing, urinary hesitancy or retention, constipation, abdominal pain, abdominal distention, nausea and vomiting, loss of libido, and impotence. Anhidrosis may produce heat intolerance and impairment of temperature regulation in a hot environment. Dysphagia, paralytic ileus, acute angle closure glaucoma, maculopapular rash, petechial rash, and scarletiniform rash have also been reported. Adverse cardiac reactions, including arrhythmias and myocardial infarction, have been reported with atropine [see Warnings and Precautions (5.1) and Clinical Pharmacology (12.2)].
Larger doses of atropine may produce central nervous system effects such as restlessness, tremor, fatigue, locomotor difficulties, delirium, and hallucinations [see Overdosage (10.1)].
Hypersensitivity reactions will occasionally occur, are usually seen as skin rashes, and may progress to exfoliation. Anaphylactic reaction and laryngospasm are rare.
6.2 Pralidoxime Chloride
Pralidoxime can cause blurred vision, diplopia and impaired accommodation, dizziness, headache, drowsiness, nausea, tachycardia, increased systolic and diastolic blood pressure [see Clinical Pharmacology (12.2)], muscular weakness, dry mouth, emesis, rash, dry skin, hyperventilation, decreased renal function, and decreased sweating when given parenterally to normal adult volunteers who have not been exposed to anticholinesterase poisons.
In several cases of organophosphorus poisoning, excitement and manic behavior have occurred immediately following recovery of consciousness, in either the presence or absence of pralidoxime administration. However, similar behavior has not been reported in subjects given pralidoxime in the absence of organophosphorus poisoning.
Elevations in AST and/or ALT enzyme levels were observed in 1 of 6 normal adult volunteers given 1200 mg of pralidoxime intramuscularly, and in 4 of 6 adult volunteers given 1800 mg intramuscularly. Levels returned to normal in about two weeks. Transient elevations in creatine kinase were observed in all normal volunteers given the drug.
6.4 Inadvertent Injection
In cases where DuoDote is inadvertently administered to people who are not poisoned with nerve agent or organophosphorus insecticide, the following effects on their ability to function normally may occur.
- Atropine 2 mg IM, roughly the equivalent of one DuoDote autoinjector, when given to healthy male volunteers, is associated with minimal effects on visual, motor, and mental functions, though unsteadiness walking and difficulty concentrating may occur. Atropine reduces body sweating and increases body temperature, particularly with exercise and under hot conditions.
- Atropine 4 mg IM, roughly the equivalent of two DuoDote autoinjectors, when given to healthy male volunteers, is associated with impaired visual acuity, visual near point accommodation, logical reasoning, digital recall, learning, and cognitive reaction time. Ability to read is reduced or lost. Subjects are unsteady and need to concentrate on walking. These effects begin about 15 minutes to one hour or more post-dose.
- Atropine 6 mg IM, roughly the equivalent of three DuoDote autoinjectors, when given to healthy male volunteers, is associated with the effects described above plus additional central effects including poor coordination, poor attention span, and visual hallucinations (colored flashes) in many subjects. Frank visual hallucinations, auditory hallucinations, disorientation, and ataxia occur in some subjects. Skilled and labor-intense tasks are performed more slowly and less efficiently. Decision making takes longer and is sometimes impaired.
It is unclear if the above data, obtained from studies of healthy adult subjects, can be extrapolated to other populations. In the elderly and patients with co-morbid conditions, the effects of ≥2 mg atropine on the ability to see, walk, and think properly are unstudied; effects may be greater in susceptible populations.
Patients who are mistakenly injected with DuoDote should avoid potentially dangerous overheating, avoid vigorous physical activity, and seek medical attention as soon as feasible.
-
7 DRUG INTERACTIONS
7.1 Succinylcholine and Mivacurium
Since pralidoxime in DuoDote reactivates cholinesterases and succinylcholine and mivacurium are metabolized by cholinesterases, patients with organophosphorus nerve agent or organophosphorus insecticide poisoning who have received DuoDote may exhibit accelerated reversal of the neuromuscular blocking effects of succinylcholine and mivacurium (relative to poisoned patients who have not received pralidoxime). Monitor for neuromuscular effects with concomitant administration.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Atropine readily crosses the placental barrier and enters fetal circulation. There are no adequate data on the developmental risk associated with the use of atropine, pralidoxime, or the combination in pregnant women. Adequate animal reproduction studies have not been conducted with atropine, pralidoxime, or the combination. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.8.2 Lactation
Risk Summary
Atropine has been reported to be excreted in human milk. It is not known whether pralidoxime is excreted in human milk. There are no data on the effects of atropine or pralidoxime on the breastfed infant or the effects of the drugs on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for DuoDote and any potential adverse effects on the breastfed infant from DuoDote or from the underlying maternal condition.8.4 Pediatric Use
Safety and effectiveness of atropine in DuoDote in patients weighing more than 41 kg (90 pounds) is supported by published literature. Safety and effectiveness of pralidoxime chloride in DuoDote in patients more than 41 kg (90 pounds) is supported by data from pharmacokinetic studies in adults and experience in the pediatric population. Adverse events seen in pediatric patients treated with atropine are similar to those that occur in adult patients, although central nervous system complaints are often seen earlier and at lower doses [see Adverse Reactions (6.1)].
Safety and effectiveness of DuoDote in pediatric patients weighing less than or equal to 41 kg (90 pounds) have not been established.
8.5 Geriatric Use
Geriatric patients may be more susceptible to the effects of atropine [see Clinical Pharmacology (12.3)].
8.6 Renal Impairment
Pralidoxime chloride can cause decreased renal function [see Adverse Reactions (6.2)]. Patients with severe renal impairment may require less frequent doses after the initial dose.
-
10 OVERDOSAGE
10.1 Symptoms
Atropine
Manifestations of atropine overdose are dose-related and include flushing, dry skin and mucous membranes, tachycardia, widely dilated pupils that are poorly responsive to light, blurred vision, and fever (which can sometimes be dangerously elevated). Locomotor difficulties, disorientation, hallucinations, delirium, confusion, agitation, coma, and central depression can occur and may last 48 hours or longer. In instances of severe atropine intoxication, respiratory depression, coma, circulatory collapse, and death may occur.Pralidoxime
It may be difficult to differentiate adverse events caused by pralidoxime from those caused by organophosphorus poisoning. Symptoms of pralidoxime overdose may include dizziness, blurred vision, diplopia, headache, impaired accommodation, nausea, and tachycardia. Transient hypertension caused by pralidoxime may last several hours.10.2 Treatment
For atropine overdose, supportive treatment should be administered. If respiration is depressed, artificial respiration with oxygen is necessary. Ice bags, a hypothermia blanket, or other methods of cooling may be required to reduce atropine-induced fever, especially in pediatric patients. Catheterization may be necessary if urinary retention occurs. Since atropine elimination largely takes place through the kidney, urinary output must be maintained and increased if possible; intravenous fluids may be indicated. Because of atropine-induced photophobia, the room should be darkened.
A benzodiazepine may be needed to control marked excitement and convulsions. However, large doses for sedation should be avoided because the central nervous system depressant effect may coincide with the depressant effect occurring late in severe atropine poisoning. Barbiturates are potentiated by the anticholinesterases; therefore, barbiturates should be used cautiously in the treatment of convulsions. Central nervous system stimulants are not recommended.
-
11 DESCRIPTION
Each prefilled DuoDote autoinjector provides a single intramuscular dose of atropine, a cholinergic muscarinic antagonist, and pralidoxime chloride, a cholinesterase reactivator, in a self-contained unit, specifically designed for administration by emergency medical services personnel.
When activated, each DuoDote autoinjector delivers the following:
- 2.1 mg of atropine in 0.7 mL of sterile, pyrogen-free solution containing 12.47 mg glycerin and not more than 2.8 mg phenol, citrate buffer, and Water for Injection. The pH range is 4.0 – 5.0.
- 600 mg of pralidoxime chloride equivalent to 476.6 mg of pralidoxime in 2 mL of sterile, pyrogen-free solution containing 40 mg benzyl alcohol, 22.5 mg glycine, and Water for Injection. The pH is adjusted with hydrochloric acid. The pH range is 2.0 to 3.0.
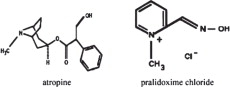
Atropine occurs as white crystals, usually needle-like, or as a white, crystalline powder. It is slightly soluble in water with a molecular weight of 289.38. Atropine, a naturally occurring belladonna alkaloid, is a racemic mixture of equal parts of d- and l-hyoscyamine, with activity due almost entirely to the levo isomer of the drug.
Chemically, atropine is designated as 1αH,5αH-Tropan-3α-ol(±)-tropate. Its empirical formula is C17H23NO3 and its structural formula is as follows:
Pralidoxime chloride is an odorless, white to pale-yellow crystalline powder, freely soluble in water, with a molecular weight of 172.61. Chemically, pralidoxime chloride is designated as 2-formyl-l-methylpyridinium chloride oxime. Its empirical formula is C7H9ClN2O and its structural formula is indicated above.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Atropine
Atropine competitively blocks the effects of acetylcholine, including excess acetylcholine due to organophosphorus poisoning, at muscarinic cholinergic receptors on smooth muscle, cardiac muscle, secretory gland cells, and in peripheral autonomic ganglia and the central nervous system.Pralidoxime
Pralidoxime reactivates acetylcholinesterase which has been inactivated by phosphorylation due to an organophosphorus nerve agent or insecticide. However, pralidoxime does not reactivate acetylcholinesterase inactivated by all organophosphorus nerve agents (e.g., soman). Pralidoxime cannot reactivate phosphorylated acetylcholinesterases that have undergone a further chemical reaction known as “aging.” Reactivated acetylcholinesterase hydrolyzes excess acetylcholine resulting from organophosphorus poisoning to help restore impaired cholinergic neural function. Reactivation is clinically important because only a small proportion of active acetylcholinesterase is needed to maintain vital functions.12.2 Pharmacodynamics
Atropine
Atropine reduces secretions in the mouth and respiratory passages, relieves airway constriction, and may reduce centrally-mediated respiratory paralysis. In severe organophosphorus poisoning, a fully atropinized patient may develop or continue to have respiratory failure and may require artificial respiration and suctioning of airway secretions. Atropine may cause thickening of secretions.Atropine-induced parasympathetic inhibition may be preceded by a transient phase of stimulation, especially on the heart where small doses first slow the rate before characteristic tachycardia develops due to paralysis of vagal control. Atropine increases heart rate and reduces atrioventricular conduction time. Adequate atropine doses can prevent or abolish bradycardia or asystole produced by organophosphorus nerve agents.
Atropine may decrease the degree of partial heart block which can occur after organophosphorus poisoning. In some patients with complete heart block, atropine may accelerate the idioventricular rate; in others, the rate is stabilized. In some patients with conduction defects, atropine may cause paradoxical atrioventricular (A-V) block and nodal rhythm.
Atropine will not act on the neuromuscular junction and has no effect on muscle paralysis or weakness, fasciculations or tremors; pralidoxime is intended to treat these symptoms.
Systemic doses of atropine slightly raise systolic and lower diastolic pressures and can produce significant postural hypotension. Such doses also slightly increase cardiac output and decrease central venous pressure. Atropine can dilate cutaneous blood vessels, particularly the “blush” area (atropine flush), and may inhibit sweating, thereby causing hyperthermia, particularly in a warm environment or with exercise [see Warnings and Precautions (5.2)].
Pralidoxime Chloride
Pralidoxime chloride has its most critical effect in relieving respiratory muscle paralysis. Because pralidoxime is less effective in relieving depression of the respiratory center, atropine is always required concomitantly to block the effect of accumulated acetylcholine at this site. Pralidoxime has a minor role in relieving muscarinic signs and symptoms, such as salivation or bronchospasm.DuoDote temporarily increases blood pressure, a known effect of pralidoxime. In a study of 24 healthy young adults administered a single dose of atropine and pralidoxime autoinjector intramuscularly (approximately 9 mg/kg pralidoxime chloride), diastolic blood pressure increased from baseline by 11 ± 14 mm Hg (mean ± SD), and systolic blood pressure increased by 16 ± 19 mm Hg, at 15 minutes post-dose. Blood pressures remained elevated at these approximate levels through one hour post-dose, began to decrease at two hours post-dose and were near pre-dose baseline at four hours post-dose.
12.3 Pharmacokinetics
Atropine:
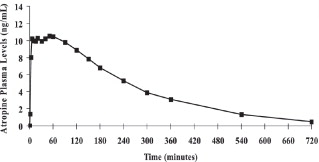
Atropine is well absorbed after intramuscular administration. Single dose DuoDote pharmacokinetic data for atropine are shown in Figure 1. The intramuscular injection site was the antero-lateral thigh.Mean atropine plasma concentrations shown in Figure 1 indicate a plateau beginning at about 5 minutes post-dose and extending through 60 minutes post-dose.
Figure 1. Mean Atropine Plasma Concentrations After a Single DuoDote Intramuscular Injection, Which Delivers 2.1 mg of Atropine Base and 600 mg Pralidoxime Chloride, N=24 Healthy Adult Subjects [Men (n=12), Women (n=12)].
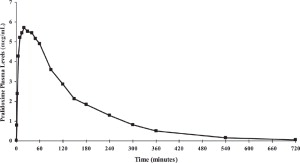
Pralidoxime Chloride:
Pralidoxime chloride is rapidly absorbed after intramuscular injection. DuoDote single dose pharmacokinetic data for pralidoxime chloride 600 mg are provided in Figure 2.Figure 2. Mean Pralidoxime Plasma Concentrations After a Single DuoDote Intramuscular Injection, Which Delivers 2.1 mg of Atropine and 600 mg Pralidoxime Chloride, N=24 Healthy Adult Subjects.
The pharmacokinetic properties of the components of DuoDote are presented in Table 2.
Table 2. Pharmacokinetic Properties of the Components of DuoDote Following Intramuscular Administration in Healthy Subjects Pharmacokinetics related to: Atropine Pralidoxime Absorption Cmax (mean ± standard deviation) 13 ± 3 ng/mL 7 ± 3 mcg/mL Tmax (mean ± standard deviation) 31 ± 30 minutes 28 ± 15 minutes Distribution Protein binding 14 to 22% to plasma proteins Not appreciable bound to serum proteins Elimination T½ 2.4 ± 0.3 hours 2 ± 1 hours Major route of excretion Urinary Urinary Percentage of dose excreted in urine 50 to 60% as unchanged drug. About 17 to 28% eliminated in the first 100 minutes. 72 to 94% as unchanged drug. About 57 to 70% eliminated in the first 30 minutes, partly as metabolite. Specific Populations
Renal and Hepatic Impairment
The pharmacokinetics of atropine or pralidoxime have not been evaluated in subjects with renal or hepatic impairment.Gender
Atropine: DuoDote AUC0-inf and Cmax values for atropine are 15% higher in females than males. The half-life of atropine is approximately 20 minutes shorter in females than males.Pralidoxime Chloride: A single DuoDote injection produced a mean Cmax for pralidoxime about 36% higher in females than males. Tmax is 23 minutes in females and 32 minutes in males. Pralidoxime half-life in males and females are 153 and 107 minutes, respectively.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
DuoDote is indicated for short-term emergency use only, and no adequate studies regarding the carcinogenic potential of atropine or pralidoxime chloride have been conducted.Mutagenesis
Studies to assess the mutagenic potential of atropine or pralidoxime chloride have not been conducted.Impairment of Fertility
Atropine:
In studies in which male rats were orally administered atropine (62.5 to 125 mg/kg) for one week prior to mating and throughout a 5-day mating period with untreated females, a dose-related decrease in fertility was observed. A no-effect dose for male reproductive toxicity was not established. The lowest dose tested was 290 times (on a mg/m2 basis) the dose of atropine in a single application of DuoDote (2.1 mg).Fertility studies of atropine in females have not been conducted.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Each single-dose DuoDote (atropine and pralidoxime chloride) autoinjector contains atropine (2.1 mg/0.7 mL; colorless to yellow solution, visible in front chamber) and pralidoxime chloride (600 mg/2 mL; colorless to yellow solution, not visible in rear chamber) and is available in a single unit carton, NDC-11704-620-01.
Each DuoDote autoinjector is supplied in a pouch that provides protection from light.
Store at 25ºC (77ºF); excursions permitted between 15ºC and 30ºC (between 59ºF and 86ºF) [See USP Controlled Room Temperature]. Not made with natural rubber latex. Keep from freezing. Protect from light.
-
17 PATIENT COUNSELING INFORMATION
Use by Healthcare Providers
DuoDote is intended for use by Healthcare Providers. See the illustrated Instruction Sheet for Healthcare Providers. -
INSTRUCTIONS FOR USE
Instruction Sheet for
Healthcare ProvidersDuoDote should be administered by healthcare providers who have had adequate training in the recognition and treatment of nerve agent or insecticide intoxication.
Individuals should not rely solely upon atropine and pralidoxime to provide complete protection from chemical nerve agents and insecticide poisoning.
Primary protection against exposure to chemical nerve agents and insecticide poisoning is the wearing of protective garments including masks designed specifically for this use.
Evacuation and decontamination procedures should be undertaken as soon as possible. Medical personnel assisting evacuated victims of nerve agent poisoning should avoid contaminating themselves by exposure to the victim's clothing.
DuoDote is indicated for the treatment of poisoning by organophosphorus nerve agents as well as organophosphorus insecticides in adults and pediatric patients weighing more than 41 kg (90 pounds). DuoDote should only be administered to patients experiencing symptoms of organophosphorus poisoning in a situation where exposure is known or suspected. DuoDote should be administered as soon as symptoms of organophosphorus poisoning appear.
The number of DuoDote autoinjectors to administer to an individual is based on severity of symptoms. Common symptoms of organophosphorus exposure are listed below. Individuals may not have all symptoms:
Mild Symptoms Severe Symptoms - Blurred vision, miosis
- Excessive, unexplained teary eyes
- Excessive, unexplained runny nose
- Increased salivation such as sudden drooling
- Chest tightness or difficulty breathing
- Tremors throughout the body or muscular twitching
- Nausea and/or vomiting
- Unexplained wheezing, coughing or increased airway secretions
- Acute onset of stomach cramps
- Tachycardia or bradycardia
- Strange or confused behavior
- Severe difficulty breathing or copious secretions from lungs/airway
- Severe muscular twitching and general weakness
- Involuntary urination and defecation
- Convulsions
- Unconsciousness
Dosage for Mild Symptoms in Adults and Pediatric Patients Weighing More Than 41 kg (90 Pounds)
First Dose: Administer one (1) DuoDote injection into the mid-lateral thigh if the patient experiences two or more mild symptoms of nerve agent or insecticide exposure.Trained healthcare providers with mild symptoms may self-administer a single dose of DuoDote.
Wait 10 to 15 minutes for DuoDote to take effect. If, after 10 to 15 minutes, the patient does not develop any of the severe symptoms listed above, no additional DuoDote injections are recommended, but definitive medical care should ordinarily be sought immediately. For healthcare providers who have self-administered DuoDote, an individual decision will need to be made to determine their capacity to continue to provide emergency care.
Additional Doses: If, at any time after the first dose, the patient develops any of the severe symptoms listed above, administer two (2) additional DuoDote injections in rapid succession, and immediately seek definitive medical care.
Dosage for Severe Symptoms in Adults and Pediatric Patients Weighing More Than 41 kg (90 Pounds)
If a patient has any of the severe symptoms listed above, immediately administer three (3) DuoDote injections into the patient's mid-lateral thigh in rapid succession, and immediately seek definitive medical care.Emergency care of the severely poisoned individual should include removal of oral and bronchial secretions, maintenance of a patent airway, supplemental oxygen, and, if necessary, artificial ventilation.
An anticonvulsant such as a benzodiazepine may be administered to treat convulsions if suspected in the unconscious individual. The effects of nerve agents and some insecticides can mask the motor signs of a seizure.
Close supervision of all severely poisoned patients is indicated for at least 48 to 72 hours.
-
INSTRUCTIONS FOR USE
- 1) Tear open the plastic pouch at any of the notches. Remove the DuoDote autoinjector from the pouch.
- 2) Place the DuoDote autoinjector in your dominant hand. (If you are right-handed, your right hand is dominant.) Firmly grasp the center of the DuoDote autoinjector with the Green Tip (needle end) pointing down.
- 3) With your other hand, pull off the Gray Safety Release. DuoDote is now ready to be administered.
- 4) The injection site is the mid-lateral thigh area. The DuoDote autoinjector can inject through clothing. However, make sure pockets at the injection site are empty.
People who may not have a lot of fat at the injection site should also be injected in the mid-lateral thigh, but before giving the injection, bunch up the thigh to provide a thicker area of injection.- 5) Firmly push the Green Tip straight down (at a 90° angle) against the mid-lateral thigh. Continue to firmly push until you feel the DuoDote autoinjector trigger.
IMPORTANT: After the autoinjector triggers, hold the DuoDote autoinjector firmly in place against the injection site for approximately 10 seconds.
- 6) Remove the DuoDote autoinjector from the thigh and look at the Green Tip. If the needle is visible, the drug has been administered. If the needle is not visible, check to be sure the Gray Safety Release has been removed, and then repeat above steps beginning with Step 4, but push harder in Step 5.
- 7) After the drug has been administered, push the needle against a hard surface to bend the needle back against the DuoDote autoinjector.
- 8) Put the used DuoDote autoinjector back into the plastic pouch, if available. Leave used DuoDote autoinjector(s) with the patient to allow other medical personnel to see the number of DuoDote autoinjector(s) administered.
- 9) Immediately move yourself and the patient away from the contaminated area and seek definitive medical care for the patient.
DuoDote® is a registered trademark of:
Meridian Medical Technologies®, Inc.
Columbia, MD 21046
A Pfizer Company
1-800-438-1985
© 2016 by Meridian Medical Technologies, Inc., a Pfizer company
Revised: 10/2017
0002022 -
PRINCIPAL DISPLAY PANEL
Principal Display Panel - DuoDote Carton Label
For use in
NERVE AGENT
or INSECTICIDE
POISONINGFor adults and pediatric
patients weighing41 kg +
or 90 lb +
NDC: 11704-620-01
DuoDote® AUTO-INJECTOR
(atropine and pralidoxime chloride injection)
Each auto-injector delivers an intramuscular injection of
2.1 mg of atropine and 600 mg of pralidoxime chloride
equivalent to 476.6 mg of pralidoximeStore at 25°C (77°F). Excursions permitted to 15-30°C (59-86°F).
Keep from freezing. Protect from light.
Rx Only
11704-62001
-
INGREDIENTS AND APPEARANCE
DUODOTE
atropine and pralidoxime chloride kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 11704-620 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 11704-620-01 1 in 1 CARTON; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) 09/28/2006 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 SYRINGE, PLASTIC 0.7 mL Part 2 1 SYRINGE, PLASTIC 2 mL Part 1 of 2 DUODOTE AUTO-INJECTOR
atropine injectionProduct Information Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength atropine (UNII: 7C0697DR9I) (atropine - UNII:7C0697DR9I) atropine 2.1 mg in 0.7 mL Inactive Ingredients Ingredient Name Strength Glycerin (UNII: PDC6A3C0OX) Citric Acid Monohydrate (UNII: 2968PHW8QP) Phenol (UNII: 339NCG44TV) Water (UNII: 059QF0KO0R) Sodium Citrate (UNII: 1Q73Q2JULR) Nitrogen (UNII: N762921K75) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 1 in 1 CARTON 1 1 in 1 POUCH 1 0.7 mL in 1 SYRINGE, PLASTIC; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021983 09/28/2006 Part 2 of 2 DUODOTE AUTO-INJECTOR
pralidoxime chloride injectionProduct Information Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength pralidoxime chloride (UNII: 38X7XS076H) (pralidoxime - UNII:P7MU9UTP52) pralidoxime chloride 600 mg in 2 mL Inactive Ingredients Ingredient Name Strength Benzyl Alcohol (UNII: LKG8494WBH) Glycine (UNII: TE7660XO1C) Water (UNII: 059QF0KO0R) Hydrochloric Acid (UNII: QTT17582CB) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 1 in 1 CARTON 1 1 in 1 POUCH 1 2 mL in 1 SYRINGE, PLASTIC; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021983 09/28/2006 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021983 09/28/2006 Labeler - Meridian Medical Technologies, Inc. (167671341) Establishment Name Address ID/FEI Business Operations Meridian Medical Technologies , Inc. 167671341 MANUFACTURE(11704-620) , LABEL(11704-620) , PACK(11704-620) , ANALYSIS(11704-620) Establishment Name Address ID/FEI Business Operations Meridian Medical Technologies, Inc. 078808315 MANUFACTURE(11704-620) , LABEL(11704-620) , PACK(11704-620) Establishment Name Address ID/FEI Business Operations Meridian Medical Technologies, Inc. 038889234 MANUFACTURE(11704-620) , ANALYSIS(11704-620)
Trademark Results [DuoDote]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 DUODOTE 78910285 3667914 Live/Registered |
Meridian Medical Technologies, Inc. 2006-06-16 |
 DUODOTE 77297826 3734693 Live/Registered |
Meridian Medical Technologies, Inc. 2007-10-05 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.