Carisoprodol, Aspirin and Codeine Phosphate Tablets USP, CIII Rx Only
Carisoprodol, Aspirin and Codeine Phosphate by
Drug Labeling and Warnings
Carisoprodol, Aspirin and Codeine Phosphate by is a Prescription medication manufactured, distributed, or labeled by Rising Pharmaceuticals, Inc., Ingenus Pharmaceuticals NJ, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
CARISOPRODOL, ASPIRIN AND CODEINE PHOSPHATE- carisoprodol, aspirin and codeine phosphate tablet
Rising Pharmaceuticals, Inc.
----------
Carisoprodol, Aspirin and Codeine Phosphate Tablets USP, CIII
Rx Only
WARNING: ADDICTION, ABUSE, AND MISUSE; RISK EVALUATION AND MITIGATION STRATEGY (REMS); LIFE-THREATENING RESPIRATORY DEPRESSION; ACCIDENTAL INGESTION; ULTRA-RAPID METABOLISM OF CODEINE AND OTHER RISK FACTORS FOR LIFE-THREATENING RESPIRATORY DEPRESSION IN CHILDREN; NEONATAL OPIOID WITHDRAWAL SYNDROME; INTERACTIONS WITH DRUGS AFFECTING CYTOCHROME P450 ISOENZYMES; and RISKS FROM CONCOMITANT USE WITH BENZODIAZEPINES OR OTHER CNS DEPRESSANTS
Addiction, Abuse and Misuse
Carisoprodol, Aspirin and Codeine Phosphate Tablets expose patients and other users to the risks of opioid addiction, abuse and misuse, which can lead to overdose and death. Assess each patient’s risk prior to prescribing Carisoprodol, Aspirin and Codeine Phosphate Tablets, and monitor all patients regularly for the development of these behaviors and conditions (see WARNINGS).
Opioid Analgesic Risk Evaluation and Mitigation Strategy (REMS)
To ensure that the benefits of opioid analgesics outweigh the risks of addiction, abuse, and misuse, the Food and Drug Administration (FDA) has required a REMS for these products (see WARNINGS). Under the requirements of the REMS, drug companies with approved opioid analgesic products must make REMS-compliant education programs available to healthcare providers. Healthcare providers are strongly encouraged to
- complete a REMS-compliant education program,
- counsel patients and/or their caregivers, with every prescription, on safe use, serious risks, storage, and disposal of these products,
- emphasize to patients and their caregivers the importance of reading the Medication Guide every time it is provided by their pharmacist, and
- consider other tools to improve patient, household, and community safety.
Life-Threatening Respiratory Depression
Serious, life-threatening, or fatal respiratory depression may occur with use of Carisoprodol, Aspirin and Codeine Phosphate Tablets. Monitor for respiratory depression, especially during initiation of Carisoprodol, Aspirin and Codeine Phosphate Tablets or following a dose increase (see WARNINGS).
Accidental Ingestion
Accidental ingestion of even one dose of Carisoprodol, Aspirin and Codeine Phosphate Tablets, especially by children, can result in a fatal overdose of Carisoprodol, Aspirin and Codeine Phosphate Tablets (see WARNINGS).
Ultra-Rapid Metabolism of Codeine and Other Risk Factors for Life-threatening Respiratory Depression in Children
Life-threatening respiratory depression and death have occurred in children who received codeine. Most of the reported cases occurred following tonsillectomy and/or adenoidectomy, and many of the children had evidence of being an ultra-rapid metabolizer of codeine due to a CYP2D6 polymorphism (see WARNINGS). Carisoprodol, Aspirin and Codeine Phosphate Tablets are contraindicated in children younger than 12 years of age and in children younger than 18 years of age following tonsillectomy and/or adenoidectomy (see CONTRAINDICATIONS). Avoid the use of Carisoprodol, Aspirin and Codeine Phosphate Tablets in adolescents 12 to 18 years of age who have other risk factors that may increase their sensitivity to the respiratory depressant effects of codeine.
Neonatal Opioid Withdrawal Syndrome
Prolonged use of Carisoprodol, Aspirin and Codeine Phosphate Tablets during pregnancy can result in neonatal opioid withdrawal syndrome, which may be life-threatening if not recognized and treated, and requires management according to protocols developed by neonatology experts. If opioid use is required for a prolonged period in a pregnant woman, advise the patient of the risk of neonatal opioid withdrawal syndrome and ensure that appropriate treatment will be available (see WARNINGS).
Interactions with Drugs Affecting Cytochrome P450 Isoenzymes
The effects of concomitant use or discontinuation of cytochrome P450 3A4 inducers, 3A4 inhibitors, or 2D6 inhibitors with codeine are complex. Use of cytochrome P450 3A4 inducers, 3A4 inhibitors, or 2D6 inhibitors with Carisoprodol, Aspirin and Codeine Phosphate Tablets requires careful consideration of the effects on codeine, and the active metabolite, morphine. (See WARNINGS, PRECAUTIONS; Drug Interactions).
Risks From Concomitant Use With Benzodiazepines Or Other CNS Depressants
Concomitant use of opioids with benzodiazepines or other central nervous system (CNS) depressants, including alcohol, may result in profound sedation, respiratory depression, coma, and death (see WARNINGS, PRECAUTIONS; Drug Interactions).
- Reserve concomitant prescribing of Carisoprodol, Aspirin and Codeine Phosphate Tablets and benzodiazepines or other CNS depressants for use in patients for whom alternative treatment options are inadequate.
- Limit dosages and durations to the minimum required.
- Follow patients for signs and symptoms of respiratory depression and sedation.
DESCRIPTION
Carisoprodol, Aspirin and Codeine Phosphate Tablets, USP is a fixed-dose combination product containing the following three products:
- 200 mg of carisoprodol, a centrally-acting muscle relaxant
- 325 mg of aspirin, an analgesic with antipyretic and anti-inflammatory properties
- 16 mg of codeine phosphate, a centrally-acting narcotic analgesic
It is available as a round, two-layered yellow and white tablet for oral administration.
Carisoprodol: Chemically, carisoprodol is N-isopropyl-2-methyl-2-propyl-1,3 propanediol dicarbamate and its molecular formula is C12H24N2O4, with a molecular weight of 260.34. The structural formula of carisoprodol is:

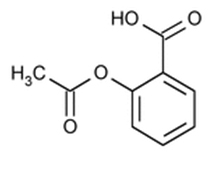
Aspirin: Chemically, aspirin (acetylsalicylic acid) is 2-(acetyloxy)-, benzoic acid and its molecular formula is C9H8O4, with a molecular weight of 180.16. The structural formula of aspirin is:
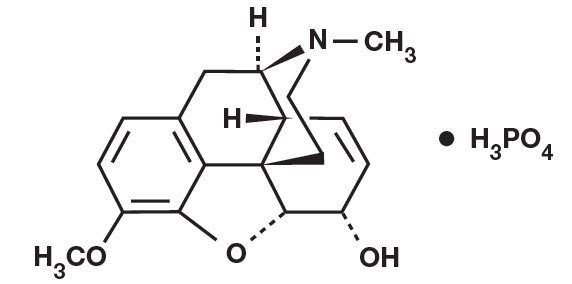
Codeine Phosphate: Chemically, codeine phosphate is 7,8 Didehydro-4,5α-epoxy-3methoxy-17- ethylmorphinan-6α-ol phosphate (1:1) (salt) hemihydrate and its molecular formula is C18H24NO7P, with a molecular weight of 406.37. The structural formula of codeine phosphate is:
Each tablet, for oral administration, contains carisoprodol 200 mg, aspirin 325 mg, and codeine phosphate 16 mg. In addition, each tablet contains the following inactive ingredients: FD&C Yellow #5 Aluminum Lake, Corn starch, Hydroxypropyl Cellulose, Lactose Anhydrous, Microcrystalline Cellulose, Magnesium Stearate, Pregelatinized Starch, Sodium Starch Glycolate and Sodium Lauryl Sulfate.
CLINICAL PHARMACOLOGY
Mechanism of Action
Carisoprodol: The mechanism of action of carisoprodol in relieving discomfort associated with acute painful musculoskeletal conditions has not been clearly identified. In animal studies, muscle relaxation induced by carisoprodol is associated with altered interneuronal activity in the spinal cord and in the descending reticular formation of the brain.
Aspirin: Aspirin is a nonsteroidal anti-inflammatory drug and a non-selective irreversible inhibitor of cyclooxygenases.
The mechanism of action of aspirin in relieving pain is by inhibition of the body’s production of prostaglandins, which are thought to cause pain sensations by stimulating muscle contractions and dilating blood vessels.
Codeine Phosphate: Codeine is an opioid agonist relatively selective for the mu-opioid receptor, but with a much weaker affinity than morphine. The analgesic properties of codeine have been speculated to come from its conversion to morphine, although the exact mechanism of analgesic action remains unknown.
Pharmacodynamics
Effects on the Central Nervous System
Codeine produces respiratory depression by direct action on brain stem respiratory centers. The respiratory depression involves a reduction in the responsiveness of the brain stem respiratory centers to both increases in carbon dioxide tension and electrical stimulation.
Codeine causes miosis, even in total darkness. Pinpoint pupils are a sign of opioid overdose but are not pathognomonic (e.g., pontine lesions of hemorrhagic or ischemic origins may produce similar findings). Marked mydriasis rather than miosis may be seen due to hypoxia in overdose situations.
Aspirin works by inhibiting the body’s production of prostaglandins, including prostaglandins involved in inflammation. Prostaglandins cause pain sensations by stimulating muscle contractions and dilating blood vessels throughout the body. In the CNS, aspirin works on the hypothalamus heat-regulating center to reduce fever, however, other mechanisms may be involved. Carisoprodol is a centrally-acting muscle relaxant that does not directly relax tense skeletal muscles. A metabolite of carisoprodol, meprobamate, has anxiolytic and sedative properties. The degree to which these properties of meprobamate contribute to the safety and efficacy of Carisoprodol, Aspirin and Codeine Phosphate Tablets is unknown.
Effects on the Gastrointestinal Tract and Other Smooth Muscle
Codeine causes a reduction in motility associated with an increase in smooth muscle tone in the antrum of the stomach and duodenum. Digestion of food in the small intestine is delayed and propulsive contractions are decreased. Propulsive peristaltic waves in the colon are decreased, while tone may be increased to the point of spasm, resulting in constipation. Other opioid-induced effects may include a reduction in biliary and pancreatic secretions, spasm of sphincter of Oddi, and transient elevations in serum amylase.
Aspirin can produce gastrointestinal injury (lesions, ulcers) through a mechanism that is not yet completely understood, but may involve a reduction in eicosanoid synthesis by the gastric mucosa. Decreased production of prostaglandins may compromise the defenses of the gastric mucosa and the activity of substances involved in tissue repair and ulcer healing.
Effects on the Cardiovascular System
Codeine produces peripheral vasodilation which may result in orthostatic hypotension or syncope. Manifestations of histamine release and/or peripheral vasodilation may include pruritus, flushing, red eyes, sweating. and/or orthostatic hypotension. Aspirin affects platelet aggregation by irreversibly inhibiting prostaglandin cyclooxygenase. This effect lasts for the life of the platelet and prevents the formation of the platelet aggregating factor, thromboxane A2. Nonacetylated salicylates do not inhibit this enzyme and have no effect on platelet aggregation. At somewhat higher doses, aspirin reversibly inhibits the formation of prostaglandin 12 (prostacyclin), which is an arterial vasodilator and inhibits platelet aggregation.
Effects on the Endocrine System
Opioids inhibit the secretion of adrenocorticotropic hormone (ACTH), cortisol, and luteinizing hormone (LH) in humans (see Adverse Reactions). They also stimulate prolactin, growth hormone (GH) secretion, and pancreatic secretion of insulin and glucagon. Chronic use of opioids may influence the hypothalamic-pituitary-gonadal axis, leading to androgen deficiency that may manifest as low libido, impotence, erectile dysfunction, amenorrhea, or infertility. The causal role of opioids in the clinical syndrome of hypogonadism is unknown because the various medical, physical, lifestyle, and psychological stressors that may influence gonadal hormone levels have not been adequately controlled for in studies conducted to date (see ADVERSE REACTIONS).
Effects on the Immune System
Opioids have been shown to have a variety of effects on components of the immune system in in vitro and animal models. The clinical significance of these findings is unknown. Overall, the effects of opioids appear to be modestly immunosuppressive.
Concentration–Efficacy Relationships
The minimum effective analgesic concentration will vary widely among patients, especially among patients who have been previously treated with potent agonist opioids. The minimum effective analgesic concentration of codeine for any individual patient may increase over time due to an increase in pain, the development of a new pain syndrome and/or the development of analgesic tolerance (see DOSAGE AND ADMINISTRATION).
Concentration–Adverse Reaction Relationships
There is a relationship between increasing codeine plasma concentration and increasing frequency of dose related opioid adverse reactions such as nausea, vomiting, CNS effects, and respiratory depression. In opioid-tolerant patients, the situation may be altered by the development of tolerance to opioid-related adverse reactions (see DOSAGE AND ADMINISTRATION).
Pharmacokinetics
Carisoprodol
The pharmacokinetics of carisoprodol and its metabolite meprobamate were studied in a study of 24 healthy subjects (12 male and 12 female) who received single doses of 350 mg of carisoprodol (see Table 1). The Cmax of meprobamate was 2.5 ± 0.5 mcg/mL (mean ± SD) after administration of a single 350 mg dose of carisoprodol, which is approximately 30% of the Cmax of meprobamate (approximately 8 mcg/mL) after administration of a single 400 mg dose of meprobamate.
| Carisoprodol | Meprobamate | |
| Cmax (µg/mL) | 1.8 ± 1 | 2.5 ± 0.5 |
| AUCinf (µghour/mL) | 7 ± 5 | 46 ± 9 |
| Tmax (hour) | 1.7 ± 0.8 | 4.5 ± 1.9 |
| T1/2 (hour) | 2 ± 0.5 | 9.6 ± 1.5 |
Absorption: Absolute bioavailability of carisoprodol has not been determined. After administration of a single dose of 350 mg of carisoprodol, the mean time to peak plasma concentrations (Tmax) of carisoprodol was approximately 1.5 to 2 hours. Co-administration of a high-fat meal with 350 mg of carisoprodol had no effect on the pharmacokinetics of carisoprodol.
Metabolism: The major pathway of carisoprodol metabolism is via the liver by cytochrome enzyme CYP2C19 to form meprobamate. This enzyme exhibits genetic polymorphism (see Patients with Reduced CYP2C19 Activity below).
Elimination: Carisoprodol is eliminated by both renal and non-renal routes with a terminal elimination half-life of approximately 2 hours after administration of a single dose of 350 mg of carisoprodol. The half-life of meprobamate is approximately 10 hours after administration of a single dose of 350 mg of carisoprodol.
Gender: Exposure of carisoprodol is higher in females than in male subjects (approximately 30 to 50% on a weight adjusted basis). Overall exposure of meprobamate is comparable between female and male subjects.
Patients with Reduced CYP2C19 Activity: Carisoprodol should be used with caution in patients with reduced CYP2C19 activity. Published studies indicate that patients who are poor CYP2C19 metabolizers have a 4-fold increase in exposure to carisoprodol, and 50% reduced exposure to meprobamate compared to normal CYP2C19 metabolizers. The prevalence of poor metabolizers in Caucasians and African Americans is approximately 3 to 5% and in Asians is approximately 15 to 20%.
Aspirin
Absorption: The rate of aspirin absorption from the gastrointestinal (GI) tract is dependent upon the presence or absence of food, gastric pH (the presence or absence of GI antacids), and other physiologic factors. Following absorption, aspirin is hydrolyzed to salicylic acid in the gut wall and during first-pass metabolism with peak plasma levels of salicylic acid occurring within 1 to 2 hours of dosing.
Distribution: Salicylic acid is widely distributed to all tissues and fluids in the body including the central nervous system (CNS), breast milk, and fetal tissues. The highest concentrations are found in the plasma, liver, kidneys, heart, and lungs. The protein binding of salicylate is concentration dependent, i.e., nonlinear. At plasma concentrations of salicylic acid, < 100 mcg/mL and > 400 mcg/mL, approximately 90 and 76 percent of plasma salicylate is bound to albumin, respectively.
Metabolism: Aspirin, which has a half-life of about 15 minutes, is hydrolyzed in the plasma to salicylic acid such that plasma levels of aspirin may not be detectable 1 to 2 hours after dosing. Salicylic acid, which has a plasma half-life of approximately 6 hours, is conjugated in the liver to form salicyluric acid, salicyl phenolic glucuronide, salicyl acyl glucuronide, gentisic acid, and gentisuric acid. At higher serum concentrations of salicylic acid, the total clearance of salicylic acid decreases due to the limited ability of the liver to form both salicyluric acid and phenolic glucuronide. Following toxic doses of aspirin (e.g., > 10 grams), the plasma half-life of salicylic acid may be increased to over 20 hours.
Elimination: The elimination of salicylic acid is constant in relation to the plasma salicylic acid concentration. Following therapeutic doses of aspirin, approximately 75, 10, 10, and 5 percent is found excreted in the urine as salicyluric acid, salicylic acid, a phenolic glucuronide of salicylic acid, and an acyl glucuronide of salicylic acid, respectively. As the urinary pH rises above 6.5, the renal clearance of free salicylate increases from less than 5 percent to greater than 80 percent. Alkalinization of the urine is a key concept in the management of salicylate overdose (see OVERDOSAGE, Treatment of Overdosage). Clearance of salicylic acid is also reduced in patients with renal impairment.
Codeine Phosphate
Absorption: Codeine is readily absorbed from the GI tract. At therapeutic doses, the analgesic effect reaches a peak within 2 hours and persists between 4 and 6 hours.
Distribution: Codeine is rapidly distributed from the intravascular spaces to the tissues with preferential uptake by the liver, spleen, and kidney. Codeine crosses the blood-brain barrier, and is found in fetal tissue and breast milk. The plasma concentration of codeine does not correlate with brain concentration of codeine or the relief of pain.
Metabolism: The plasma half-life of codeine is about 2.9 hours.
Elimination: The elimination of codeine is primarily via the kidneys, and about 90% of an oral dose is excreted by the kidneys within 24 hours of dosing. The urinary secretion products consist of free and glucuronide-conjugated codeine (about 70%), free and conjugated norcodeine (about 10%), free and conjugated morphine (about 10%), normorphine (4%), and hydrocodone (1%). The remainder of the dose is excreted in the feces.
INDICATIONS AND USAGE
Carisoprodol, Aspirin and Codeine Phosphate Tablets, USP are indicated for the relief of discomfort associated with acute, painful musculoskeletal conditions in adults.
Limitations of Use
Because of the risks of addiction, abuse, and misuse with opioids, even at recommended doses (seeWARNINGS), reserve Carisoprodol, Aspirin and Codeine Phosphate Tablets for use in patients for whom alternative treatment options (e.g., non-opioid analgesics):
- Have not been tolerated, or are not expected to be tolerated,
- Have not provided adequate analgesia, or are not expected to provide adequate analgesia
Carisoprodol, Aspirin and Codeine Phosphate Tablets,USP should only be used for short periods (up to two or three weeks) because adequate evidence of effectiveness for more prolonged use has not been established and because acute, painful musculoskeletal conditions are generally of short duration (see DOSAGE AND ADMINISTRATION).
CONTRAINDICATIONS
Carisoprodol, Aspirin and Codeine Phosphate Tablets are contraindicated for:
- All children younger than 12 years of age (see WARNINGS)
- Post-operative pain management in children of any age who have undergone tonsillectomy and/or adenoidectomy (see WARNINGS)
Carisoprodol, Aspirin and Codeine Phosphate Tablets are also contraindicated in patients with:
- Significant respiratory depression (see WARNINGS)
- Acute or severe bronchial asthma in an unmonitored setting or in the absence of resuscitative equipment (see WARNINGS)
- Concurrent use of monoamine oxidase inhibitors (MAOIs) or use of MAOIs within the last 14 days (see WARNINGS and PRECAUTIONS; Drug Interactions).
- Known or suspected gastrointestinal obstruction, including paralytic ileus (see WARNINGS)
- Hypersensitivity to codeine, aspirin, or a carbamate such as meprobamate (e.g., anaphylaxis)
- Hemophilia (see WARNINGS)
- Reye’s Syndrome (see WARNINGS)
- Known allergy to nonsteroidal anti-inflammatory drugs (NSAIDs) (see WARNINGS)
- Syndrome of asthma, rhinitis, and nasal polyps (see WARNINGS)
- Acute intermittent porphyria
WARNINGS
Addiction, Abuse, and Misuse
Carisoprodol, Aspirin, and Codeine Phosphate Tablets contains codeine and carisoprodol. Codeine in combination with carisoprodol and aspirin is a Schedule III controlled substance. As Carisoprodol, Aspirin, and Codeine Phosphate Tablets contains carisoprodol and codeine, it exposes users to the risks of addiction, abuse, and misuse (see DRUG ABUSE AND DEPENDENCE).
Although the risk of addiction in any individual is unknown, it can occur in patients appropriately prescribed Carisoprodol, Aspirin, and Codeine Phosphate Tablets. Addiction can occur at recommended dosages and if the drug is misused or abused.
Assess each patient’s risk for addiction, abuse, or misuse prior to prescribing Carisoprodol, Aspirin, and Codeine Phosphate Tablets, and monitor all patients receiving Carisoprodol, Aspirin, and Codeine Phosphate Tablets for the development of these behaviors and conditions. Risks are increased in patients with a personal or family history of substance abuse (including drug or alcohol abuse or addiction) or mental illness (e.g., major depression). The potential for these risks should not, however, prevent the proper management of pain in any given patient.
Patients at increased risk may be prescribed opioids such as Carisoprodol, Aspirin, and Codeine Phosphate Tablets, but use in such patients necessitates intensive counseling about the risks and proper use of Carisoprodol, Aspirin, and Codeine Phosphate Tablets along with intensive monitoring for signs of addiction, abuse, and misuse.
Opioids and carisoprodol are sought by drug abusers and people with addiction disorders and are subject to criminal diversion. Consider these risks when prescribing or dispensing Carisoprodol, Aspirin, and Codeine Phosphate Tablets. Strategies to reduce these risks include prescribing the drug in the smallest appropriate quantity and advising the patient on the proper disposal of unused drug (see PRECAUTIONS; Information for Patients). Contact local state professional licensing board or state controlled substances authority for information on how to prevent and detect abuse or diversion of this product.
Opioid Analgesic Risk Evaluation and Mitigation Strategy (REMS)
To ensure that the benefits of opioid analgesics outweigh the risks of addiction, abuse, and misuse, the Food and Drug Administration (FDA) has required a Risk Evaluation and Mitigation Strategy (REMS) for these products. Under the requirements of the REMS, drug companies with approved opioid analgesic products must make REMS-compliant education programs available to healthcare providers. Healthcare providers are strongly encouraged to do all of the following:
- Complete a REMS-compliant education program offered by an accredited provider of continuing education (CE) or another education program that includes all the elements of the FDA Education Blueprint for Health Care Providers Involved in the Management or Support of Patients with Pain.
- Discuss the safe use, serious risks, and proper storage and disposal of opioid analgesics with patients and/or their caregivers every time these medicines are prescribed. The Patient Counseling Guide (PCG) can be obtained at this link: www.fda.gov/OpioidAnalgesicREMSPCG.
- Emphasize to patients and their caregivers the importance of reading the Medication Guide that they will receive from their pharmacist every time an opioid analgesic is dispensed to them.
- Consider using other tools to improve patient, household, and community safety, such as patient-prescriber agreements that reinforce patient-prescriber responsibilities.
To obtain further information on the opioid analgesic REMS and for a list of accredited REMS CME/CE, call 800-503-0784, or log on to www.opioidanalgesicrems.com. The FDA Blueprint can be found at www.fda.gov/OpioidAnalgesicREMSBlueprint.
Life-Threatening Respiratory Depression
Serious, life-threatening, or fatal respiratory depression has been reported with the use of opioids, even when used as recommended. Respiratory depression, if not immediately recognized and treated, may lead to respiratory arrest and death. Management of respiratory depression may include close observation, supportive measures, and use of opioid antagonists, depending on the patient’s clinical status (see OVERDOSAGE). Carbon dioxide (CO2) retention from opioid-induced respiratory depression can exacerbate the sedating effects of opioids.
While serious, life-threatening, or fatal respiratory depression can occur at any time during the use of Carisoprodol, Aspirin, and Codeine Phosphate Tablets, the risk is greatest during the initiation of therapy or following a dosage increase. Monitor patients closely for respiratory depression, especially within the first 24-72 hours of initiating therapy with and following dosage increases of Carisoprodol, Aspirin, and Codeine Phosphate Tablets.
To reduce the risk of respiratory depression, proper dosing and titration of Carisoprodol, Aspirin, and Codeine Phosphate Tablets are essential (see DOSAGE AND ADMINISTRATION).
Overestimating the Carisoprodol, Aspirin, and Codeine Phosphate Tablets dosage when converting patients from another opioid product can result in a fatal overdose with the first dose.
Accidental ingestion of Carisoprodol, Aspirin, and Codeine Phosphate Tablets, especially by children, can result in respiratory depression and death due to an overdose of codeine and carisoprodol.
Ultra-Rapid Metabolism of Codeine and Other Risk Factors for Life-threatening Respiratory Depression in Children
Life-threatening respiratory depression and death have occurred in children who received codeine. Codeine is subject to variability in metabolism based upon CYP2D6 genotype (described below), which can lead to an increased exposure to the active metabolite morphine. Based upon post-marketing reports, children less than 12 years old appear to be more susceptible to the respiratory depressant effects of codeine, particularly if there are risk factors for respiratory depression. For example, many reported cases of death occurred in the post-operative period following tonsillectomy and/or adenoidectomy, and many of the children had evidence of being ultra-rapid metabolizers of codeine. Furthermore, children with obstructive sleep apnea who are treated with codeine for post-tonsillectomy and/or adenoidectomy pain may be particularly sensitive to its respiratory depressant effect. Because of the risk of life-threatening respiratory depression and death:
- Carisoprodol, Aspirin and Codeine Phosphate Tablets are contraindicated for all children younger than 12 years of age (see CONTRAINDICATIONS).
- Carisoprodol, Aspirin and Codeine Phosphate Tablets are contraindicated for post-operative management in pediatric patients younger than 18 years of age following tonsillectomy and/or adenoidectomy (see CONTRAINDICATIONS).
- Avoid the use of Carisoprodol, Aspirin and Codeine Phosphate Tablets in adolescents 12 to 18 years of age who have other risk factors that may increase their sensitivity to the respiratory depressant effects of codeine unless the benefits outweigh the risks. Risk factors include conditions associated with hypoventilation, such as postoperative status, obstructive sleep apnea, severe pulmonary disease, neuromuscular disease, and concomitant use of other medications that cause respiratory depression.
- As with adults, when prescribing codeine for adolescents, healthcare providers should choose the lowest effective dose for the shortest period of time and inform patients and caregivers about these risks and the signs of morphine overdose (see PRECAUTIONS; Pediatric Use,OVERDOSAGE).
Nursing Mothers
At least one death was reported in a nursing infant who was exposed to high levels of morphine in breast milk because the mother was an ultra-rapid metabolizer of codeine. Breastfeeding is not recommended during treatment with Carisoprodol, Aspirin and Codeine Phosphate Tablets (see PRECAUTIONS; Nursing Mothers).
CYP2D6 Genetic Variability: Ultra-rapid metabolizer
Some individuals may be ultra-rapid metabolizers because of a specific CYP2D6 genotype (gene duplications denoted as *1/*1xN or *1/*2xN). The prevalence of this CYP2D6 phenotype varies widely and has been estimated at 1 to 10% for Whites (European, North American), 3 to 4% for Blacks (African Americans), 1 to 2% for East Asians (Chinese, Japanese, Korean), and may be greater than 10% in certain ethnic groups (i.e., Oceanian, Northern African, Middle Eastern, Ashkenazi Jews, Puerto Rican). These individuals convert codeine into its active metabolite, morphine, more rapidly and completely than other people. This rapid conversion results in higher than expected serum morphine levels. Even at labeled dosage regimens, individuals who are ultra-rapid metabolizers may have life-threatening or fatal respiratory depression or experience signs of overdose (such as extreme sleepiness, confusion, or shallow breathing) (see OVERDOSAGE). Therefore, individuals who are ultra-rapid metabolizers should not use codeine.
Neonatal Opioid Withdrawal Syndrome
Prolonged use of Carisoprodol, Aspirin and Codeine Phosphate Tablets during pregnancy can result in withdrawal in the neonate. Neonatal opioid withdrawal syndrome, unlike opioid withdrawal syndrome in adults, may be life-threatening if not recognized and treated, and requires management according to protocols developed by neonatology experts. Observe newborns for signs of neonatal opioid withdrawal syndrome and manage accordingly. Advise pregnant women using opioids for a prolonged period of the risk of neonatal opioid withdrawal syndrome and ensure that appropriate treatment will be available (see PRECAUTIONS; Pregnancy,Information for Patients).
Risks of Interactions with Drugs Affecting Cytochrome P450 Isoenzymes
The effects of concomitant use or discontinuation of cytochrome P450 3A4 inducers, 3A4 inhibitors, or 2D6 inhibitors with codeine are complex. Use of cytochrome P450 3A4 inducers, 3A4 inhibitors, or 2D6 inhibitors with Carisoprodol, Aspirin, and Codeine Phosphate Tablets requires careful consideration of the effects on codeine and the active metabolite, morphine.
-
Cytochrome P450 3A4 Interaction
The concomitant use of Carisoprodol, Aspirin, and Codeine Phosphate Tablets with all cytochrome P450 3A4 inhibitors, such as macrolide antibiotics (e.g., erythromycin), azole-antifungal agents (e.g., ketoconazole), and protease inhibitors (e.g., ritonavir) or discontinuation of a cytochrome P450 3A4 inducer such as rifampin, carbamazepine, and phenytoin, may result in an increase in codeine plasma concentrations with subsequently greater metabolism by cytochrome P450 2D6, resulting in greater morphine levels, which could increase or prolong adverse reactions and may cause potentially fatal respiratory depression.
The concomitant use of Carisoprodol, Aspirin, and Codeine Phosphate Tablets with all cytochrome P450 3A4 inducers or discontinuation of a cytochrome P450 3A4 inhibitor may result in lower codeine levels, greater norcodeine levels, and less metabolism via 2D6 with resultant lower morphine levels. This may be associated with a decrease in efficacy, and in some patients, may result in signs and symptoms of opioid withdrawal.
Follow patients receiving Carisoprodol, Aspirin, and Codeine Phosphate Tablets and any CYP3A4 inhibitor or inducer for signs and symptoms that may reflect opioid toxicity and opioid withdrawal when Carisoprodol, Aspirin, and Codeine Phosphate Tablets is used in conjunction with inhibitors and inducers of CYP3A4.
If concomitant use of a CYP3A4 inhibitor is necessary or if a CYP3A4 inducer is discontinued, consider dosage reduction of Carisoprodol, Aspirin, and Codeine Phosphate Tablets until stable drug effects are achieved. Monitor patients for respiratory depression and sedation at frequent intervals.
If concomitant use of a CYP3A4 inducer is necessary or if a CYP3A4 inhibitor is discontinued, consider increasing the Carisoprodol, Aspirin, and Codeine Phosphate Tablets dosage until stable drug effects are achieved. Monitor for signs of opioid withdrawal. (see PRECAUTIONS; Drug Interactions). -
Risks of Concomitant Use or Discontinuation of Cytochrome P450 2D6 Inhibitors
The concomitant use of Carisoprodol, Aspirin, and Codeine Phosphate Tablets with all cytochrome P450 2D6 inhibitors (e.g., amiodarone, quinidine) may result in an increase in codeine plasma concentrations and a decrease in active metabolite morphine plasma concentration which could result in an analgesic efficacy reduction or symptoms of opioid withdrawal.
Discontinuation of a concomitantly used cytochrome P450 2D6 inhibitor may result in a decrease in codeine plasma concentration and an increase in active metabolite morphine plasma concentration which could increase or prolong adverse reactions and may cause potentially fatal respiratory depression.
Follow patients receiving Carisoprodol, Aspirin, and Codeine Phosphate Tablets and any CYP2D6 inhibitor for signs and symptoms that may reflect opioid toxicity and opioid withdrawal when Carisoprodol, Aspirin, and Codeine Phosphate Tablets are used in conjunction with inhibitors of CYP2D6.
If concomitant use with a CYP2D6 inhibitor is necessary, follow the patient for signs of reduced efficacy or opioid withdrawal and consider increasing the Carisoprodol, Aspirin, and Codeine Phosphate Tablets dosage. After stopping use of a CYP2D6 inhibitor, consider reducing the Carisoprodol, Aspirin, and Codeine Phosphate Tablets dosage and follow the patient for signs and symptoms of respiratory depression or sedation (see PRECAUTIONS; Drug Interactions).
Risks from Concomitant Use with Benzodiazepines or Other CNS Depressants
Profound sedation, respiratory depression, coma, and death may result from the concomitant use of Carisoprodol, Aspirin, and Codeine Phosphate Tablets with benzodiazepines or other CNS depressants (e.g., non-benzodiazepine sedatives/hypnotics, anxiolytics, tranquilizers, muscle relaxants, general anesthetics, antipsychotics, other opioids, alcohol). Because of these risks, reserve concomitant prescribing of these drugs for use in patients for whom alternative treatment options are inadequate.
Observational studies have demonstrated that concomitant use of opioid analgesics and benzodiazepines increases the risk of drug-related mortality compared to use of opioid analgesics alone. Because of similar pharmacological properties, it is reasonable to expect similar risk with the concomitant use of other CNS depressant drugs with opioid analgesics (see PRECAUTIONS; Drug Interactions).
If the decision is made to prescribe a benzodiazepine or other CNS depressant concomitantly with an opioid analgesic, prescribe the lowest effective dosages and minimum durations of concomitant use. In patients already receiving an opioid analgesic, prescribe a lower initial dose of the benzodiazepine or other CNS depressant than indicated in the absence of an opioid, and titrate based on clinical response. If an opioid analgesic is initiated in a patient already taking a benzodiazepine or other CNS depressant, prescribe a lower initial dose of the opioid analgesic, and titrate based on clinical response. Follow patients closely for signs and symptoms of respiratory depression and sedation.
Advise both patients and caregivers about the risks of respiratory depression and sedation when Carisoprodol, Aspirin, and Codeine Phosphate Tablets is used with benzodiazepines or other CNS depressants (including alcohol and illicit drugs). Advise patients not to drive or operate heavy machinery until the effects of concomitant use of the benzodiazepine or other CNS depressant have been determined. Screen patients for risk of substance use disorders, including opioid abuse and misuse, and warn them of the risk for overdose and death associated with the use of additional CNS depressants including alcohol and illicit drugs (see PRECAUTIONS, Drug Interactions, Information for Patients).
Life-Threatening Respiratory Depression in Patients with Chronic Pulmonary Disease or in Elderly, Cachectic, or Debilitated Patients
The use of Carisoprodol, Aspirin, and Codeine Phosphate Tablets in patients with acute or severe bronchial asthma in an unmonitored setting or in the absence of resuscitative equipment is contraindicated.
Patients with Chronic Pulmonary Disease: Carisoprodol, Aspirin, and Codeine Phosphate Tablets-treated patients with significant chronic obstructive pulmonary disease or cor pulmonale, and those with a substantially decreased respiratory reserve, hypoxia, hypercapnia, or pre-existing respiratory depression are at increased risk of decreased respiratory drive including apnea, even at recommended dosages of Carisoprodol, Aspirin, and Codeine Phosphate Tablets (see WARNINGS).
Elderly, Cachectic, or Debilitated Patients: Life-threatening respiratory depression is more likely to occur in elderly, cachectic, or debilitated patients because they may have altered pharmacokinetics or altered clearance compared to younger, healthier patients (see WARNINGS).
Monitor such patients closely, particularly when initiating and titrating Carisoprodol, Aspirin, and Codeine Phosphate Tablets and when Carisoprodol, Aspirin, and Codeine Phosphate Tablets is given concomitantly with other drugs that depress respiration (see WARNINGS).
Alternatively, consider the use of non-opioid analgesics in these patients.
Interaction with Monoamine Oxidase Inhibitors
Monoamine oxidase inhibitors (MAOIs) may potentiate the effects of morphine, codeine’s active metabolite, including respiratory depression, coma, and confusion. Carisoprodol, Aspirin, and Codeine Phosphate Tablets should not be used in patients taking MAOIs or within 14 days of stopping such treatment.
Adrenal Insufficiency
Cases of adrenal insufficiency have been reported with opioid use, more often following greater than one month of use. Presentation of adrenal insufficiency may include non-specific symptoms and signs including nausea, vomiting, anorexia, fatigue, weakness, dizziness, and low blood pressure. If adrenal insufficiency is suspected, confirm the diagnosis with diagnostic testing as soon as possible. If adrenal insufficiency is diagnosed, treat with physiologic replacement doses of corticosteroids. Wean the patient off of the opioid to allow adrenal function to recover and continue corticosteroid treatment until adrenal function recovers. Other opioids may be tried as some cases reported use of a different opioid without recurrence of adrenal insufficiency. The information available does not identify any particular opioids as being more likely to be associated with adrenal insufficiency.
Severe Hypotension
Carisoprodol, Aspirin, and Codeine Phosphate Tablets may cause severe hypotension including orthostatic hypotension and syncope in ambulatory patients. There is increased risk in patients whose ability to maintain blood pressure has already been compromised by a reduced blood volume or concurrent administration of certain CNS depressant drugs (e.g., phenothiazines or general anesthetics) (see PRECAUTIONS; Drug Interactions). Monitor these patients for signs of hypotension after initiating or titrating the dosage of Carisoprodol, Aspirin, and Codeine Phosphate Tablets. In patients with circulatory shock, Carisoprodol, Aspirin, and Codeine Phosphate Tablets may cause vasodilation that can further reduce cardiac output and blood pressure. Avoid the use of Carisoprodol, Aspirin, and Codeine Phosphate Tablets in patients with circulatory shock.
Risks of Use in Patients with Increased Intracranial Pressure, Brain Tumors, Head Injury, or Impaired Consciousness
In patients who may be susceptible to the intracranial effects of CO2 retention (e.g., those with evidence of increased intracranial pressure or brain tumors), Carisoprodol, Aspirin, and Codeine Phosphate Tablets may reduce respiratory drive, and the resultant CO2 retention can further increase intracranial pressure. Monitor such patients for signs of sedation and respiratory depression, particularly when initiating therapy with Carisoprodol, Aspirin, and Codeine Phosphate Tablets.
Opioids may also obscure the clinical course in a patient with a head injury. Avoid the use of Carisoprodol, Aspirin, and Codeine Phosphate Tablets in patients with impaired consciousness or coma.
Risks of Use in Patients with Gastrointestinal Conditions Including Peptic Ulcer Disease
Carisoprodol, Aspirin, and Codeine Phosphate Tablets is contraindicated in patients with known or suspected gastrointestinal obstruction, including paralytic ileus.
The codeine in Carisoprodol, Aspirin, and Codeine Phosphate Tablets may cause spasm of the sphincter of Oddi. Opioids may cause increases in serum amylase. Monitor patients with biliary tract disease, including acute pancreatitis for worsening symptoms.
Patients with a history of active peptic ulcer disease should avoid using aspirin, which can cause gastric mucosal irritation and bleeding.
Aspirin can cause serious gastrointestinal (GI) adverse reactions including bleeding, perforation, and obstruction of the stomach, small intestine, or large intestine, which can be fatal. Aspirin-associated serious GI adverse reactions can occur anywhere along the GI tract, at any time, with or without warning symptoms. Patients at higher risk of aspirin-associated serious upper GI adverse reactions include patients with a history of aspirin-associated GI bleeding from ulcers (complicated ulcers), a history of aspirin-associated ulcers (uncomplicated ulcers), geriatric patients, patients with poor baseline health status, patients taking higher doses of aspirin, and patients taking concomitant anticoagulants, NSAIDs, and/or large amounts of alcohol. To minimize the risk for aspirin-associated GI serious adverse reactions, the lowest effective aspirin dose should be used for the shortest possible duration.
Increased Risk of Seizures in Patients with Seizure Disorders
The codeine in Carisoprodol, Aspirin, and Codeine Phosphate Tablets may increase the frequency of seizures in patients with seizure disorders, and may increase the risk of seizures occurring in other clinical settings associated with seizures. Monitor patients with a history of seizure disorders for worsened seizure control during Carisoprodol, Aspirin, and Codeine Phosphate Tablets therapy.
There have been post-marketing reports of seizures in patients who received carisoprodol. Most of these cases have occurred in the setting of multiple drug overdoses (including drugs of abuse, illegal drugs, and alcohol) (see OVERDOSAGE).
Withdrawal
Avoid the use of mixed agonist/antagonist (e.g., pentazocine, nalbuphine, and butorphanol) or partial agonist (e.g., buprenorphine) analgesics in patients who are receiving a full opioid agonist analgesic, including Carisoprodol, Aspirin, and Codeine Phosphate Tablets. In these patients, mixed agonist/antagonist and partial agonist analgesics may reduce the analgesic effect and/or precipitate withdrawal symptoms.
When discontinuing Carisoprodol, Aspirin, and Codeine Phosphate Tablets in a physicallydependent patient, gradually taper the dosage (see DOSAGE AND ADMINISTRATION). Do not abruptly discontinue Carisoprodol, Aspirin, and Codeine Phosphate Tablets in these patients (see DRUG ABUSE AND DEPENDENCE).
Risks of Driving and Operating Machinery
Carisoprodol, Aspirin, and Codeine Phosphate Tablets may impair the mental or physical abilities needed to perform potentially hazardous activities such as driving a car or operating machinery. Warn patients not to drive or operate dangerous machinery unless they are tolerant to the effects of Carisoprodol, Aspirin, and Codeine Phosphate Tablets and know how they will react to the medication.
Coagulation Abnormalities and Bleeding Risks
Even low doses of aspirin can inhibit platelet function leading to an increase in bleeding time. This can adversely affect patients with inherited (i.e. hemophilia) or acquired (i.e. liver disease or vitamin K deficiency) bleeding disorders. Aspirin is contraindicated in patients with hemophilia.
Aspirin administered pre-operatively may prolong the bleeding time.
Patients who consume three or more alcoholic drinks every day should be counseled about the bleeding risks involved with chronic, heavy alcohol use while taking aspirin.
Reye's Syndrome
Aspirin should not be used in children or teenagers for viral infections, with or without fever, because of the risk of Reye syndrome with concomitant use of aspirin in certain viral illnesses.
Allergy
Aspirin is contraindicated in patients with known allergy to nonsteroidal anti-inflammatory drug products (NSAIDs) and in patients with the syndrome of asthma, rhinitis, and nasal polyps. Aspirin may cause severe urticaria, angioedema, or bronchospasm (asthma).
PRECAUTIONS
Information for Patients
Patients should be advised to contact their health care provider if they experience any adverse reactions to Carisoprodol, Aspirin and Codeine Phosphate Tablets. Advise the patient to read theFDA-approved patient labeling (Medication Guide).
Addiction, Abuse, and Misuse
Inform patients that the use of Carisoprodol, Aspirin, and Codeine Phosphate Tablets, even when taken as recommended, can result in addiction, abuse, and misuse, which can lead to overdose and death (see WARNINGS). Instruct patients not to share Carisoprodol, Aspirin, and Codeine Phosphate Tablets with others and to take steps to protect Carisoprodol, Aspirin, and Codeine Phosphate Tablets from theft or misuse.
Life-Threatening Respiratory Depression
Inform patients of the risk of life-threatening respiratory depression, including information that the risk is greatest when starting Carisoprodol, Aspirin, and Codeine Phosphate Tablets or when the dosage is increased, and that it can occur even at recommended dosages (see WARNINGS).Advise patients how to recognize respiratory depression and to seek medical attention if breathing difficulties develop.
Accidental Ingestion
Inform patients that accidental ingestion, especially by children, may result in respiratory depression or death. Instruct patients to take steps to store Carisoprodol, Aspirin, and Codeine Phosphate Tablets securely and to properly dispose of unused Carisoprodol, Aspirin, and Codeine Phosphate Tablets in accordance with the local state guidelines and/or regulations.
Ultra-Rapid Metabolism of Codeine and Other Risk Factors for Life-threatening Respiratory Depression in Children
Advise caregivers that Carisoprodol, Aspirin and Codeine Phosphate Tablets is contraindicated in all children younger than 12 years of age and in children younger than 18 years of age following tonsillectomy and/or adenoidectomy. Advise caregivers of children ages 12 to18 years of age receiving Carisoprodol, Aspirin, and Codeine Phosphate Tablets to monitor for signs of respiratory depression (see WARNINGS).
Interactions with Benzodiazepines and Other CNS Depressants
Inform patients and caregivers that potentially fatal additive effects may occur if Carisoprodol,Aspirin, and Codeine Phosphate Tablets is used with benzodiazepines or other CNS depressants,including alcohol, and not to use these concomitantly unless supervised by a health care provider (see WARNINGS,PRECAUTIONS; Drug Interactions).
Serotonin Syndrome
Inform patients that opioids could cause a rare but potentially life-threatening condition resulting from concomitant administration of serotonergic drugs. Warn patients of the symptoms of serotonin syndrome and to seek medical attention right away if symptoms develop. Instruct patients to inform their healthcare providers if they are taking, or plan to take serotonergic medications. (see PRECAUTIONS; Drug Interactions).
MAOI Interaction
Inform patients not to take Carisoprodol, Aspirin, and Codeine Phosphate Tablets while using any drugs that inhibit monoamine oxidase. Patients should not start MAOIs while taking Carisoprodol, Aspirin, and Codeine Phosphate Tablets (see PRECAUTIONS; Drug Interactions).
Adrenal Insufficiency
Inform patients that opioids could cause adrenal insufficiency, a potentially life-threatening condition. Adrenal insufficiency may present with non-specific symptoms and signs such as nausea, vomiting, anorexia, fatigue, weakness, dizziness, and low blood pressure. Advise patients to seek medical attention if they experience a constellation of these symptoms (see WARNINGS).
Important Administration Instructions
Instruct patients how to properly take Carisoprodol, Aspirin, and Codeine Phosphate Tablets.(see DOSAGE AND ADMINISTRATION).
Patients should take the drug only for as long as it is prescribed, in the amounts prescribed, and no more frequently than prescribed.
Hypotension
Inform patients that Carisoprodol, Aspirin, and Codeine Phosphate Tablets may cause orthostatic hypotension and syncope. Instruct patients how to recognize symptoms of low blood pressure and how to reduce the risk of serious consequences should hypotension occur (e.g., sit or lie down, carefully rise from a sitting or lying position) (see WARNINGS).
Anaphylaxis
Inform patients that anaphylaxis has been reported with ingredients contained in Carisoprodol,Aspirin, and Codeine Phosphate Tablets. Advise patients how to recognize such a reaction and when to seek medical attention (see CONTRAINDICATIONS, ADVERSE REACTIONS).
Aspirin Allergy: Patients should be informed that Carisoprodol, Aspirin, and Codeine Phosphate Tablets contains aspirin and should not be taken by patients with an aspirin or NSAIDs allergy (see WARNINGS).
Pregnancy
Neonatal Opioid Withdrawal Syndrome: Inform female patients of reproductive potential that prolonged use of Carisoprodol, Aspirin, and Codeine Phosphate Tablets during pregnancy can result in neonatal opioid withdrawal syndrome, which may be life-threatening if not recognized and treated (see WARNINGS,PRECAUTIONS; Pregnancy).
Embryo-Fetal Toxicity:Inform female patients of reproductive potential that Carisoprodol,Aspirin, and Codeine Phosphate Tablets can (or may) cause fetal harm and to inform the healthcare provider of a known or suspected pregnancy (see PRECAUTIONS; Pregnancy).
Lactation
Advise women that breastfeeding is not recommended during treatment with Carisoprodol,Aspirin, and Codeine Phosphate Tablets (see PRECAUTIONS; Nursing Mothers).
Infertility
Inform patients that chronic use of opioids may cause reduced fertility. It is not known whether these effects on fertility are reversible (see PRECAUTIONS; Females and Males of Reproductive Potential).
Risk of Bleeding
Inform patients about the signs and symptoms of bleeding. Tell patients to notify their physician if they are prescribed any drug which may increase risk of bleeding.
Counsel patients who consume three or more alcoholic drinks daily about the bleeding risks involved with chronic, heavy alcohol use while taking aspirin (see WARNINGS).
Driving or Operating Heavy Machinery
Inform patients that Carisoprodol, Aspirin, and Codeine Phosphate Tablets may impair the ability to perform potentially hazardous activities such as driving a car or operating heavy machinery. Advise patients not to perform such tasks until they know how they will react to the medication (see WARNINGS).
Constipation
Advise patients of the potential for severe constipation, including management instructions and when to seek medical attention (see ADVERSE REACTIONS).
Avoid Concomitant Use of NSAIDs
Inform patients that the concomitant use of Carisoprodol, Aspirin and Codeine Phosphate Tablets with NSAIDs or other salicylates (e.g., diflunisal, salsalate) is not recommended due to the increased risk of gastrointestinal toxicity, and little or no increase in efficacy (see WARNINGS,PRECAUTIONS; Drug Interactions). Alert patients that NSAIDs may be present in “over the counter” medications for treatment of colds, fever, or insomnia.
Disposal of Unused Carisoprodol, Aspirin, and Codeine Phosphate Tablets
Advise patients to properly dispose of unused Carisoprodol, Aspirin, and Codeine Phosphate Tablets Advise patients to throw the drug in the household trash following these steps.
- Remove them from their original containers and mix them with an undesirable substance,such as used coffee grounds or kitty litter (this makes the drug less appealing to children and pets, and unrecognizable to people who may intentionally go through the trash seeking drugs).
- Place the mixture in a sealable bag, empty can, or other container to prevent the drug from leaking or breaking out of a garbage bag, or to dispose of in accordance with the local state guidelines and/or regulations.
Carisoprodol Should Only Be Used for Short-Term Treatment
Patients should be advised that treatment with carisoprodol should be limited to acute use (up to two or three weeks) for the relief of acute, musculoskeletal discomfort. In the post-marketing experience with carisoprodol, cases of dependence, withdrawal, and abuse have been reported with prolonged use. If musculoskeletal symptoms still persist, patients should contact their healthcare provider for further evaluation.
Drug Interactions
Table 1 includes clinically significant drug interactions with Carisoprodol, Aspirin and Codeine Phosphate Tablets.
| Inhibitors of CYP3A4 | |
| Clinical Impact: | The concomitant use of Carisoprodol, Aspirin, and Codeine Phosphate Tablets with CYP3A4 inhibitors may result in an increase in codeine plasma concentrations with subsequently greater metabolism by cytochrome CYP2D6, resulting in greater morphine levels, which could increase or prolong adverse reactions and may cause potentially fatal respiratory depression, particularly when an inhibitor is added after a stable dose of Carisoprodol, Aspirin, and Codeine Phosphate Tablets is achieved (see WARNINGS). After stopping a CYP3A4 inhibitor, as the effects of the inhibitor decline,it may result in lower codeine levels, greater norcodeine levels, and less metabolism via 2D6 with resultant lower morphine levels (see CLINICAL PHARMACOLOGY; Pharmacokinetics), resulting in decreased opioid efficacy or a withdrawal syndrome in patients who had developed physical dependence to codeine. |
| Intervention: | If concomitant use with CYP3A4 inhibitor is necessary, consider dosage reduction of Carisoprodol, Aspirin, and Codeine Phosphate Tablets until stable drug effects are achieved. Monitor patients for respiratory depression and sedation at frequent intervals. If a CYP3A4 inhibitor is discontinued, consider increasing theCarisoprodol, Aspirin, and Codeine Phosphate Tablets dosage until stable drug effects are achieved. Monitor for signs of opioid withdrawal. |
| Examples: | Macrolide antibiotics (e.g., erythromycin), azole-antifungal agents (e.g.ketoconazole), protease inhibitors (e.g., ritonavir) |
| CYP3A4 Inducers | |
| Clinical Impact: | The concomitant use of Carisoprodol, Aspirin, and Codeine Phosphate Tablets and CYP3A4 inducers can result in lower codeine levels, greater norcodeine levels, and less metabolism via 2D6 with resultant lower morphine levels (see CLINICAL PHARMACOLOGY; Pharmacokinetics), resulting in decreased efficacy or onset of a withdrawal syndrome in patients who have developed physical dependence (see WARNINGS). After stopping a CYP3A4 inducer, as the effects of the inducer decline,the codeine plasma concentration may increase with subsequently greater metabolism by cytochrome CYP2D6, resulting in greater morphine levels(see CLINICAL PHARMACOLOGY; Pharmacokinetics), which could increase or prolong both the therapeutic effects and adverse reactions,and may cause serious respiratory depression. |
| Intervention: | If concomitant use of a CYP3A4 inducer is necessary, follow the patient for reduced efficacy and signs of opioid withdrawal and consider increasing the Carisoprodol, Aspirin, and Codeine Phosphate Tablets dosage as needed. If a CYP3A4 inducer is discontinued, consider Carisoprodol, Aspirin,and Codeine Phosphate Tablets dosage reduction, and monitor for signs of respiratory depression and sedation at frequent intervals. |
| Examples: | Rifampin, carbamazepine, phenytoin |
| Inhibitors of CYP2D6 | |
| Clinical Impact: | Codeine in Carisoprodol, Aspirin, and Codeine Phosphate Tablets is metabolized by CYP2D6 to form morphine. The concomitant use of Carisoprodol, Aspirin, and Codeine Phosphate Tablets and CYP2D6 inhibitors can increase the plasma concentration of codeine, but can decrease the plasma concentrations of active metabolite morphine which could result in reduced analgesic efficacy or symptoms of opioid withdrawal, particularly when an inhibitor is added after a stable dose of Carisoprodol, Aspirin, and Codeine Phosphate Tablets is achieved (see CLINICAL PHARMACOLOGY; Pharmacokinetics). After stopping a CYP2D6 inhibitor, as the effects of the inhibitor decline, the codeine plasma concentration will decrease but the active metabolite morphine plasma concentration will increase, which could increase or prolong adverse reactions and may cause potentially fatal respiratory depression (see CLINICAL PHARMACOLOGY; Pharmacokinetics). |
| Intervention: | If concomitant use with a CYP2D6 inhibitor is necessary, or if a CYP2D6 inhibitor is discontinued after concomitant use, consider dosage adjustment of Carisoprodol, Aspirin, and Codeine Phosphate Tablets and monitor patients closely at frequent intervals. If concomitant use with CYP2D6 inhibitors is necessary, follow the patient for reduced efficacy or signs and symptoms of opioid withdrawal and consider increasing the Carisoprodol, Aspirin, and Codeine Phosphate Tablets as needed. After stopping use of a CYP2D6 inhibitor, consider reducing the Carisoprodol, Aspirin, and Codeine Phosphate Tablets and monitor the patient for signs and symptoms of respiratory depression or sedation. |
| Examples: | paroxetine, fluoxetine, bupropion, quinidine |
| Benzodiazepines and other Central Nervous System (CNS) Depressants | |
| Clinical Impact: | Due to additive pharmacologic effect, the concomitant use of benzodiazepines or other CNS depressants including alcohol, increases the risk of respiratory depression, profound sedation, coma, and death. |
| Intervention: | Reserve concomitant prescribing of these drugs for use in patients for whom alternative treatment options are inadequate. Limit dosages and durations to the minimum required. Follow patients closely for signs of respiratory depression and sedation (see WARNINGS). |
| Examples: | Benzodiazepines and other sedatives/hypnotics, anxiolytics, tranquilizers, muscle relaxants, general anesthetics, antipsychotics, other opioids, alcohol. |
| Serotonergic Drugs | |
| Clinical Impact: | The concomitant use of opioids with other drugs that affect the serotonergic neurotransmitter system has resulted in serotonin syndrome. |
| Intervention: | If concomitant use is warranted, carefully observe the patient,particularly during treatment initiation and dose adjustment. DiscontinueCarisoprodol, Aspirin, and Codeine Phosphate Tablets if serotonin syndrome is suspected. |
| Examples: | Selective serotonin reuptake inhibitors (SSRIs), serotonin and norepinephrine reuptake inhibitors (SNRIs), tricyclic antidepressants(TCAs), triptans, 5-HT3 receptor antagonists, drugs that affect the serotonin neurotransmitter system (e.g., mirtazapine, trazodone,tramadol), monoamine oxidase (MAO) inhibitors (those intended to treat psychiatric disorders and also others, such as linezolid and intravenous methylene blue). |
| Monoamine Oxidase Inhibitors (MAOIs) | |
| Clinical Impact: | MAOI interactions with opioids may manifest as serotonin syndrome or opioid toxicity (e.g., respiratory depression, coma) (see WARNINGS). |
| Intervention: | Do not use Carisoprodol, Aspirin, and Codeine Phosphate Tablets in patients taking MAOIs or within 14 days of stopping such treatment. If urgent use of an opioid is necessary, use test doses and frequent titration of small doses of other opioids (such as oxycodone,hydrocodone, oxymorphone, hydrocodone, or buprenorphine) to treatpain while closely monitoring blood pressure and signs and symptoms ofCNS and respiratory depression. |
| Examples: | phenelzine, tranylcypromine, linezolid |
| Mixed Agonist/Antagonist and Partial Agonist Opioid Analgesics | |
| Clinical Impact: | May reduce the analgesic effect of Carisoprodol, Aspirin, and Codeine Phosphate Tablets and/or precipitate withdrawal symptoms. |
| Intervention: | Avoid concomitant use. |
| Examples: | butorphanol, nalbuphine, pentazocine, buprenorphine, |
| Muscle Relaxants | |
| Clinical Impact: | Codeine may enhance the neuromuscular blocking action of skeletalmuscle relaxants and produce an increased degree of respiratorydepression. |
| Intervention: | Monitor patients for signs of respiratory depression that may be greaterthan otherwise expected and decrease the dosage of Carisoprodol,Aspirin, and Codeine Phosphate Tablets and/or the muscle relaxant asnecessary. |
| Diuretics | |
| Clinical Impact: | Opioids can reduce the efficacy of diuretics by inducing the release ofantidiuretic hormone. |
| Intervention: | Monitor patients for signs of diminished diuresis and/or effects on bloodpressure and increase the dosage of the diuretic as needed.The effectiveness of diuretics in patients with underlying renal orcardiovascular disease may be diminished by the concomitantadministration of aspirin due to inhibition of renal prostaglandins, leading to decreased renal blood flow and salt and fluid retention. |
| Anticholinergic Drugs | |
| Clinical Impact: | The concomitant use of anticholinergic drugs may increase risk of urinary retention and/or severe constipation, which may lead to paralytic ileus. |
| Intervention: | Monitor patients for signs of urinary retention or reduced gastric motility when Carisoprodol, Aspirin, and Codeine Phosphate Tablets is used concomitantly with anticholinergic drugs. |
| Anticoagulants | |
| Clinical Impact: | Aspirin may enhance the effects of anticoagulants. Concurrent use may increase the risk of bleeding. Aspirin can also displace warfarin from protein binding sides, leading to prolongation of both the prothrombin time and the bleeding time. |
| Intervention: | Monitor patients for signs of bleeding. |
| Examples: | Warfarin, heparin, enoxaparin, clopidogrel, prasugrel, rivaroxaban, apixaban |
| Uricosuric Agents | |
| Clinical Impact: | Aspirin inhibits the uricosuric effects of uricosuric agents. |
| Intervention: | Avoid concomitant use. |
| Examples: | Probenecid |
| Carbonic Anhydrase Inhibitors | |
| Clinical Impact: | Concurrent use with aspirin can lead to high serum concentrations of the carbonic anhydrase inhibitor and cause toxicity due to competition at the renal tubule for secretion. |
| Intervention: | Consider reducing the dose of the carbonic anhydrase inhibitor and monitor patient for any adverse effects from the carbonic anhydrase inhibitor. |
| Examples: | Acetazolamide, methazolamide |
| Methotrexate | |
| Clinical Impact: | Aspirin may enhance the toxicity of methotrexate by displacing it from its plasma protein binding sites and/or reducing its renal clearance. |
| Intervention: | Use caution if using concomitantly, especially in elderly patients or patients with renal impairment. Monitor patients for methotrexate toxicity. |
| Nephrotoxic Agents | |
| Clinical Impact: | Concomitant use with aspirin may lead to additive nephrotoxicity due to the inhibition of renal prostaglandins by aspirin. Also, the plasma concentration of aspirin is increased by conditions that reduce the glomerular filtration rate or tubular secretion. |
| Intervention: | Use Carisoprodol, Aspirin, and Codeine Phosphate Tablets with caution if used concomitantly with nephrotoxic agents. Closely monitor the renal function of patients |
| Examples: | Aminoglycosides, amphotericin B, systemic bacitracin, cisplatin, cyclosporine, foscarnet, or parenteral vancomycin |
| Angiotensin Converting Enzyme (ACE) Inhibitors | |
| Clinical Impact: | The hyponatremic and hypotensive effects of ACE inhibitors may be diminished by the concomitant administration of aspirin due to its indirect effect on the renin-angiotensin conversion pathway. |
| Intervention: | Use caution if using concomitantly. Monitor the blood pressure and renal function of patients. |
| Examples: | Ramipril, captopril |
| Beta Blockers | |
| Clinical Impact: | The hypotensive effects of beta blockers may be diminished by the concomitant administration of aspirin due to inhibition of renal prostaglandins, leading to decreased renal blood flow, and salt and fluid retention. |
| Intervention: | Use caution if using concomitantly. Monitor the blood pressure and renal function of patients |
| Examples: | Metoprolol, propranolol |
| Hypoglycemic Agents | |
| Clinical Impact: | Aspirin may increase the serum glucose-lowering action of insulin and sulfonylureas leading to hypoglycemia. |
| Intervention: | Patients should be advised to consult a physician if any signs or symptoms of hypoglycemia occur. |
| Examples: | Insulin, glimepiride, glipizide |
| Anticonvulsants | |
| Clinical Impact: | Aspirin can displace protein-bound phenytoin and valproic acid, leading to a decrease in the total concentration of phenytoin and an increase in serum valproic acid levels. |
| Intervention: | Use caution if using concomitantly |
| Examples: | Phenytoin, valproic acid |
| Nonsteroidal Anti-inflammatory Drugs (NSAIDs) | |
| Clinical Impact: | Concurrent use with aspirin may increase the risk of bleeding or lead to decreased renal function. Aspirin may enhance serious side effects and toxicity of ketorolac by displacing it from its plasma protein binding sites and/or reducing its renal clearance. |
| Intervention: | Avoid concomitant use |
| Examples: | Ketorolac, ibuprofen, naproxen, diclofenac |
| Corticosteroids | |
| Clinical Impact: | In patients receiving concomitant corticosteroids and chronic use of aspirin, withdrawal of corticosteroids may result in salicylism because corticosteroids enhance renal clearance of salicylates and their withdrawal is followed by return to normal rates of renal clearance. |
| Intervention: | Avoid concomitant use |
Drug/Drug Interactions with Carisoprodol
The sedative effect of carisoprodol and other CNS depressants (e.g., alcohol, benzodiazepines, opioids, tricyclic antidepressants) may be additive. Therefore, caution should be exercised with patients who take more than one of these CNS depressants simultaneously. Concomitant use of carisoprodol and meprobamate, a metabolite of carisoprodol, is not recommended (see WARNINGS).
Carisoprodol is metabolized in the liver by CYP2C19 to form meprobamate (see CLINICAL PHARMACOLOGY). Co-administration of CYP2C19 inhibitors, such as omeprazole or fluvoxamine, with carisoprodol could result in increased exposure of carisoprodol and decreased exposure of meprobamate. Co-administration of CYP2C19 inducers, such as rifampin or St. John's Wort, with carisoprodol could result in decreased exposure of carisoprodol and increased exposure of meprobamate. Low dose aspirin also showed an induction effect on CYP2C19. The full pharmacological impact of these potential alterations of exposures in terms of either efficacy or safety of carisoprodol is unknown.
Drug/Laboratory Test Interactions
Aspirin: Aspirin may interfere with the following laboratory determinations in blood: serum amylase, fasting blood glucose, cholesterol, protein, serum glutamic-oxalacetic transaminase (SGOT), uric acid, prothrombin time and bleeding time. Aspirin may interfere with the following laboratory determinations in urine: glucose, 5-hydroxy-indoleacetic acid, Gerhardt ketone, vanillylmandelic acid (VMA), uric acid, diacetic acid, and spectrophotometric detection of barbiturates.
Codeine: Codeine may increase serum amylase levels.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Caricinogenesis
Long term studies in animals have not been performed to evaluate the carcinogenic potential of Carisoprodol, Aspirin, and Codeine Phosphate Tablets.
Administration of aspirin for 68 weeks at 0.5 percent in the feed of rats was not carcinogenic.
Two-year carcinogenicity studies with codeine sulfate have been conducted in F344/N rats and B6C3F1 mice. There was no evidence of carcinogenicity in male and female rats, respectively, at dietary doses up to 70 and 80 mg/kg/day of codeine sulfate (approximately 4 times the maximum recommended daily dose of 180 mg/day for adults on a mg/m2 basis) for two years. Similarly there was no evidence of carcinogenicity activity in male and female mice at dietary doses up to 400 mg/kg/day of codeine sulfate (approximately 10 times the maximum recommended daily dose of 180 mg/day for adults on a mg/m2 basis) for two years.
Mutagenesis
Codeine was not mutagenic in the in vitro bacterial reverse mutation assay or clastogenic in the in vitro Chinese hamster ovary cell chromosome aberration assay.
Aspirin is not mutagenic in the Ames Salmonella assay; however, aspirin did induce chromosome aberrations in cultured human fibroblasts
Carisoprodol was not formally evaluated for genotoxicity. In published studies, carisoprodol was mutagenic in the in vitro mouse lymphoma cell assay in the absence of metabolizing enzymes, but was not mutagenic in the presence of metabolizing enzymes. Carisoprodol was clastogenic in the in vitro chromosomal aberration assay using Chinese hamster ovary cells with or without the presence of metabolizing enzymes. Other types of genotoxic tests resulted in negative findings. Carisoprodol was not mutagenic in the Ames reverse mutation assay using S. typhimuriumstrains with or without metabolizing enzymes, and was not clastogenic in an in vitro mouse micronucleus assay of circulating blood cells.
Impairment of Fertility
No adequate studies have been conducted in animals to characterize the impact of the combinations of carisoprodol, aspirin, and codeine on fertility. There are also no data on codeine alone.
Aspirin inhibits ovulation in rats.
Carisoprodol was not formally evaluated for effects on fertility. Published reproductive studies of carisoprodol in mice found no alteration in fertility although an alteration in reproductive cycles characterized by a greater time spent in estrus was observed at a carisoprodol dose of 1200 mg/kg/day. In a 13-week toxicology study that did not determine fertility, mouse testes weight and sperm motility were reduced at a dose of 1200 mg/kg/day. In both studies, the no effect level was 750 mg/kg/day, corresponding to approximately 2.6 times the human equivalent dosage of 350 mg four times a day, based on a body surface area comparison.
The significance of these findings for human fertility is not known.
Pregnancy
Pregnancy Category D
Risk Summary
Prolonged use of opioid analgesics during pregnancy may cause neonatal opioid withdrawal syndrome. Use of aspirin, including Carisoprodol, Aspirin, and Codeine Phosphate Tablets, during the third trimester of pregnancy increases the risk of premature closure of the fetal ductus arteriosus. Avoid use of NSAIDs, including Carisoprodol, Aspirin, and Codeine Phosphate Tablets, in pregnant women starting at 30 weeks of gestation (third trimester).
Available data with Carisoprodol, Aspirin, and Codeine Phosphate Tablets in pregnant women are insufficient to inform a drug-associated risk for major birth defects and miscarriage. Animal reproduction studies have not been conducted with the combination of carisoproldol, aspirin and caffeine, and codeine phosphate.
In animal reproduction studies, codeine administration during organogenesis has been shown to produce delayed ossification in the offspring of mice at 2.8 times maximum recommended human dose (MRHD) of 180 mg/day, embryolethal and fetotoxic effects in the offspring of rats and hamsters at approximately 4 to 6 times the MRHD, and cranial malformations/ cranioschisis in the offspring of hamsters between 2 and 8 times the MRHD (see Data). Based on animal data, prostaglandins have been shown to have an important role in endometrial vascular permeability, blastocyst implantation and decidualization. In animal studies, administration of prostaglandin synthesis inhibitors such as aspirin, resulted in increased pre- and postimplantation loss.
Studies of aspirin use in pregnant women have not shown that aspirin increases the risk of abnormalities when administered during the first trimester of pregnancy. In controlled studies involving 41,337 pregnant women and their offspring, there was no evidence that aspirin taken during pregnancy caused stillbirth, neonatal death or reduced birth weight. In controlled studies of 50,282 pregnant women and their offspring, aspirin administration in moderate and heavy doses during the first four lunar months of pregnancy showed no teratogenic effect.
Therapeutic doses of aspirin in pregnant women close to term may cause bleeding in mother, fetus, or neonate. During the last 6 months of pregnancy, regular use of aspirin in high doses may prolong pregnancy and delivery.
There are no data on the use of carisoprodol during human pregnancy. Animal studies indicate that carisoprodol crosses the placenta and results in adverse effects on fetal growth and postnatal survival. The primary metabolite of carisoprodol, meprobamate, is an approved anxiolytic. Retrospective, post-marketing studies do not show a consistent association between maternal use of meprobamate and an increased risk for particular congenital malformations.
Animal studies have not adequately evaluated the teratogenic effects of carisoprodol. There was no increase in the incidence of congenital malformations noted in the reproductive studies in rats, rabbits, and mice treated with meprobamate. Retrospective, post-marketing studies of meprobamate during human pregnancy were equivocal for demonstrating an increased risk of congenital malformations following the first trimester exposure. Across studies that indicated an increased risk, the types of malformations were inconsistent.
In animal studies, carisoprodol reduced fetal weights, postnatal weight gain, and postnatal survival at maternal doses equivalent to 1 to 1.5 times the human dose (based on a body surface area comparison). Rats exposed to meprobamate in utero showed behavioral alterations that persisted into adulthood. For children exposed to meprobamate in utero, one study found no adverse effects on mental or motor development or IQ scores. Carisoprodol should be used during pregnancy only if the potential benefit justifies the risk to the fetus.
The background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Clinical Considerations
Fetal/Neonatal Adverse Reaction
Prolonged use of opioid analgesics during pregnancy for medical or nonmedical purposes can result in physical dependence in the neonate and neonatal opioid withdrawal syndrome shortly after birth.
Neonatal opioid withdrawal syndrome presents as irritability, hyperactivity and abnormal sleep pattern, high pitched cry, tremor, vomiting, diarrhea and failure to gain weight. The onset, duration, and severity of neonatal opioid withdrawal syndrome vary based on the specific opioid used, duration of use, timing and amount of last maternal use, and rate of elimination of the drug by the newborn. Observe newborns for symptoms of neonatal opioid withdrawal syndrome and manage accordingly (see WARNINGS).
Labor and Delivery
There are no studies on the effects of Carisoprodol, Aspirin, and Codeine Phosphate Tablets during labor or delivery. In animal studies, NSAIDS, including aspirin, inhibit prostaglandin synthesis, cause delayed parturition, and increase the incidence of stillbirth.
Opioids such as codeine cross the placenta and may produce respiratory depression and psycho-physiologic effects in neonates. An opioid antagonist, such as naloxone, must be available for reversal of opioid-induced respiratory depression in the neonate. Carisoprodol, Aspirin, and Codeine Phosphate Tablets is not recommended for use in pregnant women during or immediately prior to labor, when other analgesic techniques are more appropriate. Opioid analgesics, including Carisoprodol, Aspirin, and Codeine Phosphate Tablets, can prolong labor through actions which temporarily reduce the strength, duration, and frequency of uterine contractions. However, this effect is not consistent and may be offset by an increased rate of cervical dilation, which tends to shorten labor. Monitor neonates exposed to opioid analgesics during labor for signs of excess sedation and respiratory depression.
Aspirin should be avoided one week prior to and during labor and delivery because it can result in excessive blood loss at delivery. Prolonged gestation and prolonged labor due to prostaglandin inhibition have been reported.
Salicylates readily cross the placenta and by inhibiting prostaglandin synthesis, may cause constriction of ductus arteriosus resulting in pulmonary hypertension and increased fetal mortality and, possibly other untoward fetal effects. Aspirin use in pregnancy can also result in alteration in maternal and neonatal hemostasis mechanisms. Maternal aspirin use during later stages of pregnancy may cause low birth weight, increased incidence of intracranial hemorrhage in premature infants, stillbirths and neonatal death. Use during pregnancy, especially in the third trimester, should be avoided.
There is no information about the effects of carisoprodol on the mother and the fetus during labor and delivery.
Data
Animal Data
Animal reproduction studies have not been conducted with the combination of carisoprodol, aspirin, and codeine phosphate.
- Codeine
In a study in which pregnant hamsters were administered 150 mg/kg twice daily of codeine (oral; approximately 14 times the maximum recommended daily dose of 180 mg/day for adults on a mg/m2 basis) during organogenesis cranial malformations (i.e., meningoencephalocele) in several fetuses were reported; as well as the observation of increases in the percentage of resorptions per litter. Doses of 50 and 150 mg/kg, bid resulted in fetotoxicity as demonstrated by decreased fetal body weight. In an earlier study in hamsters, single oral doses of 73 to 360 mg/kg level on Gestation Day 8 (oral; approximately 4 to 16 times the maximum recommended daily dose of 180 mg/day for adults on a mg/m2 basis), reportedly produced cranioschisis in all of the fetuses examined.
In studies in rats, doses at the 120 mg/kg level (oral; approximately 6 times the maximum recommended daily dose of 180 mg/day for adults on a mg/m2 basis) during organogenesis, in the toxic range for the adult animal, were associated with an increase in embryo resorption at the time of implantation.
In pregnant mice, a single 100 mg/kg dose (subcutaneous; approximately 2.8 times the recommended daily dose of 180 mg/day for adults on a mg/mg2 basis) administered between Gestation Day 7 and 12 reportedly resulted in delayed ossification in the offspring.
No teratogenic effects were observed in rabbits administered up to 30 mg/kg (approximately 4 times the maximum recommended daily dose of 180 mg/day for adults on a mg/m2 basis) of codeine during organogenesis.
Codeine (30 mg/kg) administered subcutaneously to pregnant rats during pregnancy and for 25 days after delivery increased neonatal mortality at birth. This dose is 1.6 times the maximum recommended human dose of 180 mg/day on a body surface area comparison.
Nursing Mothers
Risk Summary
Codeine and its active metabolite, morphine, are present in human milk. There are published studies and cases that have reported excessive sedation, respiratory depression, and death (one case) in infants exposed to codeine via breast milk. Women who are ultra-rapid metabolizers of codeine achieve higher than expected serum levels of morphine, potentially leading to higher levels of morphine in breast milk that can be dangerous in their breastfed infants. In women with normal codeine metabolism (normal CYP2D6 activity), the amount of codeine secreted into human milk is low and dose-dependent. There is no information on the effects of the codeine on milk production. Because of the potential for serious adverse reactions, including excess sedation, respiratory depression, and death in a breastfed infant, advise patients that breastfeeding is not recommended during treatment with Carisoprodol, Aspirin and Codeine Phosphate Tablets (see WARNINGS).
The aspirin in Carisoprodol, Aspirin and Codeine Phosphate Tablets are also excreted in breast milk in small amounts. Adverse effects on platelet function in the nursing infant exposed to aspirin in breast milk may be a potential risk. Furthermore, nursing women are advised against aspirin use because of the possible development of Reye's Syndrome in their babies.
Very limited data in humans show that carisoprodol is present in breast milk and may reach concentrations two to four times the maternal plasma concentrations. In one case report, a breast-fed infant received about 4 to 6% of the maternal daily dose through breast milk and experienced no adverse effects. However, milk production was inadequate and the baby was supplemented with formula. In lactation studies in mice, female pup survival and pup weight at weaning were decreased. This information suggests that maternal use of carisoprodol may lead to reduced or less effective infant feeding (due to sedation) and/or decreased milk production. Caution should be exercised when carisoprodol is administered to a nursing woman.
Clinical Consideration
If infants are exposed to Carisoprodol, Aspirin and Codeine Phosphate Tablets through breast milk, they should be monitored for excess sedation and respiratory depression. Withdrawal symptoms can occur in breastfed infants when maternal administration of an opioid analgesic is stopped, or when breast-feeding is stopped.
Females and Males of Reproductive Potential
Infertility
Chronic use of opioids may cause reduced fertility in females and males of reproductive potential. It is not known whether these effects on fertility are reversible (see ADVERSE REACTIONS, CLINICAL PHARMACOLOGY, PRECAUTIONS; Carcinogenesis, Mutagenesis, Impairment of Fertility).
Females
Based on the mechanism of action, the use of prostaglandin-mediated NSAIDs, including aspirin, may delay or prevent rupture of ovarian follicles, which has been associated with reversible infertility in some women. Published animal studies have shown that administration of prostaglandin synthesis inhibitors has the potential to disrupt prostaglandin-mediated follicular rupture required for ovulation. Small studies in women treated with NSAIDs have also shown a reversible delay in ovulation. Consider withdrawal of NSAIDs, including aspirin, in women who have difficulties conceiving or who are undergoing investigation of infertility.
Pediatric Use
Preparations containing aspirin should be kept out of the reach of children. Reye’s Syndrome is a rare condition that affects the brain and liver and is most often observed in children given aspirin during a viral illness. The efficacy and safety of Carisoprodol, Aspirin and Codeine Phosphate Tablets in pediatric patients less than 18 years of age have not been established.
Life-threatening respiratory depression and death have occurred in children who received codeine (see WARNINGS). In most of the reported cases, these events followed tonsillectomy and/or adenoidectomy, and many of the children had evidence of being ultra-rapid metabolizers of codeine (i.e., multiple copies of the gene for cytochrome P450 isoenzyme 2D6 or high morphine concentrations). Children with sleep apnea may be particularly sensitive to the respiratory depressant effects of codeine.
Because of the risk of life-threatening respiratory depression and death:
- Carisoprodol, Aspirin and Codeine Phosphate Tablets are contraindicated for all children younger than 12 years of age (see CONTRAINDICATIONS).
- Carisoprodol, Aspirin and Codeine Phosphate Tablets are contraindicated for post-operative management in pediatric patients younger than 18 years of age following tonsillectomy and/or adenoidectomy (see CONTRAINDICATIONS).
- Avoid the use of Carisoprodol, Aspirin and Codeine Phosphate Tablets in adolescents 12 to 18 years of age who have other risk factors that may increase their sensitivity to the respiratory depressant effects of codeine unless the benefits outweigh the risks. Risk factors include conditions associated with hypoventilation, such as postoperative status, obstructive sleep apnea, obesity, severe pulmonary disease, neuromuscular disease, and concomitant use of other medications that cause respiratory depression (see WARNINGS).
Geriatric Use
Clinical studies of Carisoprodol, Aspirin, and Codeine Phosphate Tablets did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
Elderly patients (aged 65 years or older) may have increased sensitivity to Carisoprodol, Aspirin, and Codeine Phosphate Tablets. In general, use caution when selecting a dosage for an elderly patient, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function and of concomitant disease or other drug therapy.
Respiratory depression is the chief risk for elderly patients treated with opioids, and has occurred after large initial doses were administered to patients who were not opioid-tolerant or when opioids were co-administered with other agents that depress respiration. Titrate the dosage of Carisoprodol, Aspirin, and Codeine Phosphate Tablets slowly in geriatric patients and monitor closely for signs of central nervous system and respiratory depression (see WARNINGS).
Components of this product are known to be substantially excreted by the kidney, and the risk of adverse reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
Elderly patients, compared to younger patients, are at greater risk for NSAID-associated serious cardiovascular, gastrointestinal, and/or renal adverse reactions. If the anticipated benefit for the elderly patient outweighs these potential risks, dose selection should start at the low end of the dosing range, and monitor patients for adverse effects (see WARNINGS).
Hepatic Impairment
No formal studies have been conducted in patients with hepatic impairment so the pharmacokinetics of aspirin, codeine and in this patient population are unknown. Start these patients cautiously with lower doses of Carisoprodol, Aspirin, and Codeine Phosphate Tablets or with longer dosing intervals and titrate slowly while carefully monitoring for side effects. In patients with severe hepatic disease, monitor effects of therapy with serial liver function tests
Since carisoprodol is excreted by the kidney and is metabolized in the liver, caution should be exercised if carisoprodol is administered to patients with impaired hepatic function. Carisoprodol is dialyzable by hemodialysis and peritoneal dialysis.
Renal Impairment
Carisoprodol, Aspirin, and Codeine Phosphate Tablets contains aspirin, which should be avoided in patients with severe renal failure (glomerular filtration rate less than 10 mL/minute).
Codeine pharmacokinetics may be altered in patients with renal failure. Clearance may be decreased and the metabolites may accumulate to much higher plasma levels in patients with renal failure as compared to patients with normal renal function. Start these patients cautiously with lower doses of Carisoprodol, Aspirin, and Codeine Phosphate Tablets or with longer dosing intervals and titrate slowly while carefully monitoring for side effects. In patients with renal disease, monitor effects of therapy with serial renal function tests.
Since carisoprodol is excreted by the kidney and is metabolized in the liver, caution should be exercised if carisoprodol is administered to patients with impaired renal function.
Carisoprodol is dialyzable by hemodialysis and peritoneal dialysis.
ADVERSE REACTIONS
The following serious adverse reactions are described, or described in greater detail, in other sections:
- Addiction, Abuse, and Misuse (see WARNINGS)
- Life-Threatening Respiratory Depression (see WARNINGS)
- Interactions with Benzodiazepines and Other CNS Depressants (see WARNINGS)
- Ultra-Rapid Metabolism of Codeine and Other Risk Factors for Life-Threatening Respiratory Depression in Children (see WARNINGS)
- Neonatal Opioid Withdrawal Syndrome (see WARNINGS)
- Adrenal Insufficiency (see WARNINGS)
- Severe Hypotension (see WARNINGS)
- Gastrointestinal Adverse Reactions (see WARNINGS)
- Seizures (see WARNINGS)
- Withdrawal (see WARNINGS)
- Coagulation Abnormalities and Bleeding (see WARNINGS)
- Reye’s Syndrome (see WARNINGS)
- Allergy (see WARNINGS)
The following adverse reactions which have occurred with the administration of the individual products alone may also occur with the use of Carisoprodol, Aspirin and Codeine Phosphate Tablets. The following events have been reported during post-approval individual use of carisoprodol, aspirin, and codeine. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Carisoprodol
Cardiovascular: Tachycardia, postural hypotension, and facial flushing (see OVERDOSAGE).
Central Nervous System: Drowsiness, dizziness, vertigo, ataxia, tremor, agitation, irritability, headache, depressive reactions, syncope, insomnia, and seizures (see OVERDOSAGE).
Gastrointestinal: Nausea, vomiting, and epigastric discomfort.
Hematologic: Leukopenia, pancytopenia.
Aspirin
The most common adverse reactions associated with the use of aspirin have been gastrointestinal, including both abdominal pain, anorexia, nausea, vomiting, gastritis, and occult bleeding (see WARNINGS).Other adverse reactions associated with the use of aspirin include elevated liver enzymes, rash, pruritus, purpura, intracranial hemorrhage, interstitial nephritis, acute renal failure, and tinnitus. Tinnitus may be a sign of high serum salicylate levels (see OVERDOSAGE).
Codeine Phosphate
Nausea, vomiting, constipation, miosis, sedation, dizziness.
Adrenal insufficiency: Cases of adrenal insufficiency have been reported with opioid use, more often following greater than one month of use.
Anaphylaxis: Anaphylaxis has been reported with ingredients contained in Carisoprodol, Aspirin and Codeine Phosphate Tablets.
Androgen deficiency: Cases of androgen deficiency have occurred with chronic use of opioids [see CLINICAL PHARMACOLOGY].
To report SUSPECTED ADVERSE REACTIONS, contact Ingenus Pharmaceuticals NJ, LLC at 1-877-748-1970 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG ABUSE AND DEPEDENCE
Controlled Substance:
Carisoprodol, Aspirin, and Codeine Phosphate Tablets contains codeine. Codeine in combination with carisoprodol and aspirin is a Schedule III controlled substance.
Abuse
Carisoprodol, Aspirin, and Codeine Phosphate Tablets contains codeine, a substance with a high potential for abuse similar to other opioids, including fentanyl, hydrocodone, hydromorphone, methadone, morphine, oxycodone, oxymorphone, and tapentadol. Carisoprodol, Aspirin, and Codeine Phosphate Tablets can be abused and is subject to misuse, addiction, and criminal diversion (see WARNINGS).
All patients treated with opioids require careful monitoring for signs of abuse and addiction, because use of opioid analgesic products carries the risk of addiction even under appropriate medical use.
Prescription drug abuse is the intentional non-therapeutic use of a prescription drug, even once, for its rewarding psychological or physiological effects.
Drug addiction is a cluster of behavioral, cognitive, and physiological phenomena that develop after repeated substance use and includes: a strong desire to take the drug, difficulties in controlling its use, persisting in its use despite harmful consequences, a higher priority given to drug use than to other activities and obligations, increased tolerance, and sometimes a physical withdrawal.
“Drug-seeking” behavior is very common in persons with substance use disorders. Drug-seeking tactics include emergency calls or visits near the end of office hours, refusal to undergo appropriate examination, testing, or referral, repeated “loss” of prescriptions, tampering with prescriptions, and reluctance to provide prior medical records or contact information for other treating healthcare provider(s). “Doctor shopping” (visiting multiple prescribers to obtain additional prescriptions) is common among drug abusers and people suffering from untreated addiction. Preoccupation with achieving adequate pain relief can be appropriate behavior in a patient with poor pain control.
Abuse and addiction are separate and distinct from physical dependence and tolerance. Healthcare providers should be aware that addiction may not be accompanied by concurrent tolerance and symptoms of physical dependence in all addicts. In addition, abuse of opioids can occur in the absence of true addiction.
Carisoprodol, Aspirin, and Codeine Phosphate Tablets, like other opioids, can be diverted for non-medical use into illicit channels of distribution. Careful record-keeping of prescribing information, including quantity, frequency, and renewal requests, as required by state and federal law, is strongly advised.
Proper assessment of the patient, proper prescribing practices, periodic re-evaluation of therapy, and proper dispensing and storage are appropriate measures that help to limit abuse of opioid drugs.
Risks Specific to Abuse of Carisoprodol, Aspirin, and Codeine Phosphate Tablets
Carisoprodol, Aspirin, and Codeine Phosphate Tablets is for oral use only. Abuse of Carisoprodol, Aspirin, and Codeine Phosphate Tablets poses a risk of overdose and death. The risk is increased with concurrent abuse of Carisoprodol, Aspirin, and Codeine Phosphate Tablets with alcohol and other central nervous system depressants.
Parenteral drug abuse is commonly associated with transmission of infectious diseases such as hepatitis and HIV.
Carisoprodol
Abuse of carisoprodol poses a risk of overdosage which may lead to death, CNS and respiratory depression, hypotension, seizures and other disorders (see WARNINGS). Patients at high risk of carisoprodol abuse may include those with prolonged use of carisoprodol, with a history of drug abuse, or those who use carisoprodol in combination with other abused drugs.
Dependence
Both tolerance and physical dependence can develop during chronic opioid therapy. Tolerance is the need for increasing doses of opioids to maintain a defined effect such as analgesia (in the absence of disease progression or other external factors). Tolerance may occur to both the desired and undesired effects of drugs, and may develop at different rates for different effects.
Physical dependence results in withdrawal symptoms after abrupt discontinuation or a significant dosage reduction of a drug. Withdrawal also may be precipitated through the administration of drugs with opioid antagonist activity (e.g., naloxone, nalmefene), mixed agonist/antagonist analgesics (e.g., pentazocine, butorphanol, nalbuphine), or partial agonists (e.g., buprenorphine). Physical dependence may not occur to a clinically significant degree until after several days to weeks of continued opioid usage.
Carisoprodol, Aspirin, and Codeine Phosphate Tablets should not be abruptly discontinued in a physically-dependent patient (see DOSAGE AND ADMINISTRATION). If Carisoprodol, Aspirin, and Codeine Phosphate Tablets is abruptly discontinued in a physically-dependent patient, a withdrawal syndrome may occur. Some or all of the following can characterize this syndrome: restlessness, lacrimation, rhinorrhea, yawning, perspiration, chills, myalgia, and mydriasis. Other signs and symptoms also may develop, including: irritability, anxiety, backache, joint pain, weakness, abdominal cramps, insomnia, nausea, anorexia, vomiting, diarrhea, or increased blood pressure, respiratory rate, or heart rate.
Infants born to mothers physically dependent on opioids will also be physically dependent and may exhibit respiratory difficulties and withdrawal signs (see PRECAUTIONS; Pregnancy and Nursing Mothers).
OVERDOSAGE
Clinical Presentation
Acute overdose with Carisoprodol, Aspirin and Codeine Phosphate Tablets can be manifested by respiratory depression, somnolence progressing to stupor or coma, skeletal muscle flaccidity, cold and clammy skin, constricted pupils, and, in some cases, pulmonary edema, bradycardia, hypotension, partial or complete airway obstruction, atypical snoring, and death. Marked mydriasis rather than miosis may be seen with hypoxia in overdose situations [see Clinical Pharmacology].
Carisoprodol: Overdosage of carisoprodol commonly produces CNS depression. Death, coma, respiratory depression, hypotension, seizures, delirium, hallucinations, dystonic reactions, nystagmus, blurred vision, mydriasis, euphoria, muscular incoordination, rigidity, and/or headache have been reported with carisoprodol overdosage. Serotonin syndrome has been reported with carisoprodol intoxication. Many of the carisoprodol overdoses have occurred in the setting of multiple drug overdoses (including drugs of abuse, illegal drugs, and alcohol). The effects of an overdose of carisoprodol and other CNS depressants (e.g., alcohol, benzodiazepines, opioids, tricyclic antidepressants) can be additive even when one of the drugs has been taken in the recommended dosage. Fatal accidental and non-accidental overdoses of carisoprodol have been reported alone or in combination with CNS depressants.
Aspirin: Salicylate toxicity may result from an overdose of an acute ingestion or chronic intoxication. Mild to moderate salicylate poisoning is usually associated with plasma salicylic concentrations about 200 mcg/mL and is characterized by tinnitus, hearing difficulty, headache, dim vision, dizziness, tachypnea, increased thirst, nausea, vomiting, sweating, and diarrhea. In the early stages of overdose, CNS stimulation and respiratory alkalosis can occur; however, in the later stages CNS depression and metabolic acidosis can occur.
Symptoms and signs of severe salicylate poisoning, associated with plasma salicylic concentrations greater than 400 mcg/mL, include hyperthermia, dehydration, delirium, GI hemorrhage, pulmonary edema, and CNS depression (e.g., coma). Death is usually due to respiratory failure or cardiovascular collapse.
Overdose of aspirin in pediatric patients: Salicylate poisoning should be considered in pediatric patients with symptoms of vomiting, hyperpnea, and hyperthermia. Salicylate poisoning should be considered in infants with metabolic acidosis and all pediatric patients with severe salicylate poisoning.
Codeine Phosphate: Acute overdose of opioids, including codeine phosphate, is characterized by CNS depression (somnolence progressing to coma), respiratory depression, hypotension, miosis, skeletal muscle flaccidity, and cold and clammy skin.
Treatment of Overdosage
In case of overdose, priorities are the reestablishment of a patent and protected airway and institution of assisted or controlled ventilation, if needed. Employ other supportive measures (including oxygen and vasopressors) in the management of circulatory shock and pulmonary edema as indicated. Cardiac arrest or arrhythmias will require advanced life-support techniques.
The opioid antagonists, naloxone or nalmefene, are specific antidotes to respiratory depression resulting from opioid overdose. For clinically significant respiratory or circulatory depression secondary to codeine phosphate overdose, administer an opioid antagonist. Opioid antagonists should not be administered in the absence of clinically significant respiratory or circulatory depression secondary to codeine overdose.
Because the duration of opioid reversal is expected to be less than the duration of action of codeine in Carisoprodol, Aspirin and Codeine Phosphate Tablets, carefully monitor the patient until spontaneous respiration is reliably reestablished. If the response to an opioid antagonist is suboptimal or only brief in nature, administer additional antagonist as directed by the product’s prescribing information.
In an individual physically dependent on opioids, administration of the recommended usual dosage of the antagonist will precipitate an acute withdrawal syndrome. The severity of the withdrawal symptoms experienced will depend on the degree of physical dependence and the dose of the antagonist administered. If a decision is made to treat serious respiratory depression in the physically dependent patient, administration of the antagonist should be begun with care and by titration with smaller than usual doses of the antagonist.
Carisoprodol: Basic life support measures should be instituted as dictated by the clinical presentation of the carisoprodol overdose. Vomiting should not be induced due to the risk of CNS and respiratory depression, and subsequent aspiration. Gastric lavage should be considered soon after ingestion (within one hour). Circulatory support should be administered with volume infusion and vasopressor agents if needed. Seizures should be treated with intravenous benzodiazepines and the reoccurrence of seizures may be treated with phenobarbital. In cases of severe CNS depression, airway protective reflexes may be compromised and tracheal intubation should be considered for airway protection and respiratory support. For decontamination in cases of severe toxicity, activated charcoal should be considered in a hospital setting in patients with large overdoses who present early and are not demonstrating CNS depression and can protect their airway.
Aspirin: Since there are no specific antidotes for salicylate poisoning, the aim of the treatment is to enhance elimination of salicylate; reduce further salicylate absorption; correct fluid, electrolyte, or acid/base imbalances; and provide cardio-respiratory support. The acid-base status should be followed closely with serial serum pH determinations (using arterial blood gas). If acidosis is present, intravenous sodium bicarbonate should be given, along with adequate hydration, until salicylate levels decrease to within the therapeutic range. To enhance elimination, forced diuresis and alkalinization of the urine may be beneficial. Gastric emptying and/or lavage are recommended as soon as possible after ingestion, even if the patient has vomited spontaneously. After lavage and/or emesis, administration of activated charcoal is beneficial, if less than 3 hours have passed since ingestion. Charcoal absorption should not be employed prior to emesis and lavage. In patients with renal insufficiency or in cases of life-threatening aspirin intoxication, hemodialysis or peritoneal dialysis is usually required.
Additional treatment of aspirin overdose in pediatric patients: Pediatric patients should be sponged with tepid water. Infusion of glucose may be required to control hypoglycemia.
Exchange transfusion may be indicated in infants and young children.
DOSAGE AND ADMINISTRATION
Important Dosage and Administration Instructions
Use the lowest effective dosage for the shortest duration consistent with individual patient treatment goals (see WARNINGS).
Initiate the dosing regimen for each patient individually, taking into account the patient's severity of pain, patient response, prior analgesic treatment experience, and risk factors for addiction, abuse, and misuse (see WARNINGS).
Dosing Information
The recommended daily dose of Carisoprodol, Aspirin and Codeine Phosphate Tablets is 1 or 2 tablets, four times daily in adults. One Carisoprodol, Aspirin and Codeine Phosphate Tablet contains 200 mg of carisoprodol, 325 mg of aspirin, and 16 mg of codeine phosphate. The maximum daily dose (i.e., two tablets taken four times daily) will provide 1600 mg of carisoprodol, 2600 mg of aspirin, and 128 mg of codeine phosphate per day. The recommended maximum duration of Carisoprodol, Aspirin and Codeine Phosphate Tablet, USP use is up to two or three weeks.
Discontinuation of Carisoprodol, Aspirin and Codeine Phosphate Tablets
When a patient who has been taking Carisoprodol, Aspirin and Codeine Phosphate Tablets regularly and may be physically dependent no longer requires therapy with Carisoprodol, Aspirin and Codeine Phosphate Tablets, use a gradual downward titration of the dosage to prevent signs and symptoms of withdrawal. Do not stop Carisoprodol, Aspirin and Codeine Phosphate Tablets abruptly (see WARNINGS and DRUG ABUSE AND DEPENDENCE).
HOW SUPPLIED
Carisoprodol, Aspirin and Codeine Phosphate Tablets, USP are supplied as:
Yellow and white color, round unscored convex, two layered tablets debossed on yellow layer with “CL” over “024” and plain on the white layer.
The tablets are available in bottles of 100, NDC: 64980-176-01.
Storage
Store at 20° to 25°C (68° to 77°F) (See USP Controlled Room Temperature). Protect from moisture. Dispense in a tight, light-resistant container as defined in the USP, with a child-resistant closure (as required).
Manufactured for:
Rising Pharmaceuticals, Inc.
Allendale, NJ 07401 USA
Manufactured by:
Ingenus Pharmaceuticals NJ, LLC
Fairfield, NJ 07004 USA
5508 Rev.07/2018
PATIENT MEDICATION INFORMATION SECTION
Medication Guide
Carisoprodol (kar eyeʺ soe proeʹ dol), Aspirin (asʹ pir in), and Codeine Phosphate (koeʹ deen fosʹ fate) Tablets, CIII
Carisoprodol, Aspirin, and Codeine Phosphate Tablets is:
- A strong prescription pain medicine that contains an opioid (narcotic) that is indicated for the relief of discomfort associated with acute, painful musculoskeletal conditions in adults, when other pain treatments such as non-opioid pain medicines do not treat your pain well enough or you cannot tolerate them.
- An opioid pain medicine that can put you at risk for overdose and death. Even if you take your dose correctly as prescribed you are at risk for opioid addiction, abuse, and misuse that can lead to death.
Carisoprodol, Aspirin and Codeine Phosphate Tablets should only be used for up to 2 or 3 weeks. It is not known if Carisoprodol, Aspirin and Codeine Phosphate Tablets is effective when used for longer periods.
Important information about Carisoprodol, Aspirin, and Codeine Phosphate Tablets:
- Get emergency help right away if you take too much Carisoprodol, Aspirin, and Codeine Phosphate Tablets (overdose). When you first start taking Carisoprodol, Aspirin, and Codeine Phosphate Tablets, when your dose is changed, or if you take too much (overdose), serious or life-threatening breathing problems that can lead to death may occur.
- Taking Carisoprodol, Aspirin, and Codeine Phosphate Tablets with other opioid medicines, benzodiazepines, alcohol, or other central nervous system depressants (including street drugs) can cause severe drowsiness, decreased awareness, breathing problems, coma, and death.
- Never give anyone else your Carisoprodol, Aspirin, and Codeine Phosphate Tablets. They could die from taking it. Store Carisoprodol, Aspirin, and Codeine Phosphate Tablets away from children and in a safe place to prevent stealing or abuse. Selling or giving away Carisoprodol, Aspirin, and Codeine Phosphate Tablets is against the law.
Important Information Guiding Use in Pediatric Patients:
- Do not give Carisoprodol, Aspirin, and Codeine Phosphate Tablets to a child younger than 12 years of age.
- Do not give Carisoprodol, Aspirin, and Codeine Phosphate Tablets to a child younger than 18 years of age to treat pain after surgery to remove the tonsils and/or adenoids.
- Avoid giving Carisoprodol, Aspirin, and Codeine Phosphate Tablets to children between 12 to 18 years of age who have risk factors for breathing problems such as obstructive sleep apnea, obesity, or underlying lung problems.
- Do not give Carisoprodol, Aspirin, and Codeine Phosphate Tablets to a child or teenager with a viral illness. Reye syndrome, a lifethreatening condition, can happen when aspirin (an ingredient in Carisoprodol, Aspirin, and Codeine Phosphate Tablets) is used in children and teenagers who have certain viral illnesses.
Do not take Carisoprodol, Aspirin, and Codeine Phosphate Tablets if you have:
- severe asthma, trouble breathing, or other lung problems.
- a bowel blockage or have narrowing of the stomach or intestines.
- known allergy to nonsteroidal anti-inflammatory drug products (NSAIDs)
- a rare disorder in which your blood doesn’t clot normally (hemophilia)
Before taking Carisoprodol, Aspirin, and Codeine Phosphate Tablets, tell your healthcare provider if you have a history of:
- head injury, seizures
- liver, kidney, thyroid problems
- problems urinating
- pancreas or gallbladder problems
- abuse of street or prescription drugs, alcohol addiction, or mental health problems
- have been told by your healthcare provider that you are a “rapid metabolizer” of certain medicines
Tell your healthcare provider if you are:
- pregnant or planning to become pregnant. Carisoprodol, Aspirin, and Codeine Phosphate Tablets may harm your unborn baby. Prolonged use of Carisoprodol, Aspirin, and Codeine Phosphate Tablets during pregnancy can cause withdrawal symptoms in your newborn baby that could be life-threatening if not recognized and treated.
- breastfeeding. Not recommended; may harm your baby.
- taking prescription or over-the-counter medicines, vitamins, or herbal supplements. Taking Carisoprodol, Aspirin, and Codeine Phosphate Tablets with certain other medicines can cause serious side effects that could lead to death.
When taking Carisoprodol, Aspirin, and Codeine Phosphate Tablets:
- Do not change your dose. Take Carisoprodol, Aspirin, and Codeine Phosphate Tablets exactly as prescribed by your healthcare provider. Use the lowest dose possible for the shortest time needed.
- Take your prescribed dose of 1 or 2 tablets 4 times daily. Total daily dosage should not exceed 8 tablets. Do not take more than your prescribed dose. If you miss a dose, take your next dose at your usual time.
- Call your healthcare provider if the dose you are taking does not control your pain.
- If you have been taking Carisoprodol, Aspirin, and Codeine Phosphate Tablets regularly, do not stop taking Carisoprodol, Aspirin, and Codeine Phosphate Tablets without talking to your healthcare provider.
- After you stop taking Carisoprodol, Aspirin, and Codeine Phosphate Tablets, dispose the unused Carisoprodol, Aspirin, and Codeine Phosphate Tablets in accordance with the local state guidelines and/or regulations.
While taking Carisoprodol, Aspirin, and Codeine Phosphate Tablets DO NOT:
- Drive or operate heavy machinery, until you know how Carisoprodol, Aspirin, and Codeine Phosphate Tablets affects you. Carisoprodol, Aspirin, and Codeine Phosphate Tablets can make you sleepy, dizzy, or lightheaded.
- Drink alcohol or use prescription or over-the-counter medicines that contain alcohol. Using products containing alcohol during treatment with Carisoprodol, Aspirin, and Codeine Phosphate Tablets may cause you to overdose and die.
The possible side effects of Carisoprodol, Aspirin, and Codeine Phosphate Tablets:
- constipation, nausea, sleepiness, vomiting, tiredness, headache, dizziness, abdominal pain. Call your healthcare provider if you have any of these symptoms and they are severe.
Get emergency medical help if you have:
- trouble breathing, shortness of breath, fast heartbeat, chest pain, swelling of your face, tongue, or throat, extreme drowsiness, light-headedness when changing positions, feeling faint, agitation, high body temperature, trouble walking, stiff muscles, or mental changes such as confusion.
- If you are a nursing mother taking Carisoprodol, Aspirin, and Codeine Phosphate Tablets and your breastfeeding baby has increased sleepiness, confusion, difficulty breathing, shallow breathing, limpness, or difficulty breastfeeding.
These are not all the possible side effects of Carisoprodol, Aspirin, and Codeine Phosphate Tablets. Call your doctor for medical advice about side effects. You may report side effects to Ingenus Pharmaceuticals NJ, LLC at 1-877-748-1970 or FDA at 1-800-FDA-1088. For more information go to fda.report
Distributed by:
Rising Pharmaceuticals, Inc.
Allendale, NJ 07401 USA
This Medication Guide has been approved by the U.S. Food and Drug Administration. Issued:07/2018
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Rising® NDC: 64980-176-01
Carisoprodol, Aspirin
and Codeine phosphate
Tablets, USP
200 mg/325 mg/16 mg
100 Tablets Rx Only

| CARISOPRODOL, ASPIRIN AND CODEINE PHOSPHATE
carisoprodol, aspirin and codeine phosphate tablet |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - Rising Pharmaceuticals, Inc. (041241766) |
| Registrant - Ingenus Pharmaceuticals NJ, LLC (964680206) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Ingenus Pharmaceuticals NJ, LLC | 964680206 | MANUFACTURE(64980-176) | |