PHENAZOPYRIDINE HYDROCHLORIDE tablet, coated
Phenazopyridine Hydrochloride by
Drug Labeling and Warnings
Phenazopyridine Hydrochloride by is a Prescription medication manufactured, distributed, or labeled by Bryant Ranch Prepack. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- CAUTION
-
DESCRIPTION
Phenazopyridine Hydrochloride is light or dark red to dark violet, odorless, slightly bitter, crystalline powder. It has a specific local analgesic effect in the urinary tract, promptly relieving burning and pain. It has the following structural formula.
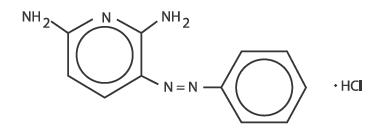
Inactive Ingredients: Corn Starch, Croscarmellose Sodium, Hypromellose, Magnesium Stearate, Microcrystalline Cellulose, Mineral Oil, Povidone, Pregelatinized Starch, Propylene Glycol and Silicon Dioxide.
- CLINICAL PHARMACOLOGY
-
INDICATIONS AND USAGE
Phenazopyridine HCl is indicated for the symptomatic relief of pain, burning, urgency, frequency, and other discomforts arising from irritation of the lower urinary tract mucosa caused by infection, trauma, surgery, endoscopic procedures, or the passage of sounds or catheters. The use of Phenazopyridine HCl for relief of symptoms should not delay definitive diagnosis and treatment of causative conditions. Because it provides only symptomatic relief, prompt appropriate treatment of the cause of pain must be instituted and Phenazopyridine HCl should be discontinued when symptoms are controlled.
The analgesic action may reduce or eliminate the need for systemic analgesics or narcotics. It is, however, compatible with antibacterial therapy and can help to relieve pain and discomfort during the interval before antibacterial therapy controls the infection. Treatment of a urinary tract infection with Phenazopyridine HCl should not exceed 2 days because there is a lack of evidence that the combined administration of Phenazopyridine HCl and an antibacterial provides greater benefit than administration of the antibacterial alone after 2 days. (See DOSAGE AND ADMINISTRATION section).
- CONTRAINDICATIONS
-
ADVERSE REACTIONS
Headache, rash, pruritus and occasional gastrointestinal disturbance. An anaphylactoid like reaction has been described. Methemoglobinemia, hemolytic anemia, renal and hepatic toxicity have been reported, usually at overdosage levels (see OVERDOSAGE Section).
-
PRECAUTIONS
General
A yellowish tinge of the skin or sclera may indicate accumulation due to impaired renal excretion and the need to discontinue therapy. The decline in renal function associated with advanced age should be kept in mind.
NOTE : Patients should be informed that Phenazopyridine HCl produces a reddish-orange discoloration of the urine and may stain fabric. Staining of contact lenses has been reported.
Laboratory Test Interaction
Due to its properties as an azo dye, Phenazopyridine HCl may interfere with urinalysis based on spectrometry or color reactions.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term administration of Phenazopyridine HCl has induced neoplasia in rats (large intestine) and mice (liver). Although no association between Phenazopyridine HCl and human neoplasia has been reported, adequate epidemiological studies along these lines have not been conducted.
Pregnancy Category B
Reproduction studies have been performed in rats at doses up to 50 mg/Kg/day and have revealed no evidence of impaired fertility or harm to the fetus due to Phenazopyridine HCl. There are, however, no adequate and well controlled studies pregnant women. Because animal production studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
-
DOSAGE AND ADMINISTRATION
100 mg Tablets: Average adult dosage is two tablets 3 times a day after meals.
200 mg Tablets: Average adult dosage is one tablet 3 times a day after meals.
When used concomitantly with an antibacterial agent for the treatment of a urinary tract infection, the administration of Phenazopyridine HCl should not exceed 2 days.
-
OVERDOSAGE
Exceeding the recommended dose in patients with good renal function or administering the usual dose to patients with impaired renal function (common in elderly patients) may lead to increased serum levels and toxic reactions. Methemoglobinemia generally follows a massive, acute overdose. Methylene blue, 1 to 2 mg/kg/body weight intravenously or ascorbic acid 100 to 200 mg given orally should cause prompt reduction of the methemoglobinemia and disappearance of the cyanosis which is an aid in diagnosis. Oxidative Heinz body hemolytic anemia may also occur, and "bite cells" (degmacytes) may be present in a chronic overdosage situation. Red blood cell G-6-PD deficiency may predispose to hemolysis. Renal and hepatic impairment and occasional failure, usually due to hypersensitivity, may also occur.
-
HOW SUPPLIED
Product: 63629-6846
NDC: 63629-6846-1 30 TABLET, COATED in a BOTTLE
NDC: 63629-6846-2 7 TABLET, COATED in a BOTTLE
NDC: 63629-6846-3 10 TABLET, COATED in a BOTTLE
NDC: 63629-6846-4 14 TABLET, COATED in a BOTTLE
NDC: 63629-6846-5 6 TABLET, COATED in a BOTTLE
Product: 63629-6844
NDC: 63629-6844-1 30 TABLET, COATED in a BOTTLE
NDC: 63629-6844-2 6 TABLET, COATED in a BOTTLE
NDC: 63629-6844-3 7 TABLET, COATED in a BOTTLE
NDC: 63629-6844-4 10 TABLET, COATED in a BOTTLE
NDC: 63629-6844-5 14 TABLET, COATED in a BOTTLE
- SPL UNCLASSIFIED SECTION
- 340B PHENAZOPYRIDINE HCL 200MG TABLET
- 340B PHENAZOPYRIDINE HCL 100MG TAB.
-
INGREDIENTS AND APPEARANCE
PHENAZOPYRIDINE HYDROCHLORIDE
phenazopyridine hydrochloride tablet, coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 63629-6846(NDC:42937-702) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PHENAZOPYRIDINE HYDROCHLORIDE (UNII: 0EWG668W17) (PHENAZOPYRIDINE - UNII:K2J09EMJ52) PHENAZOPYRIDINE HYDROCHLORIDE 200 mg Inactive Ingredients Ingredient Name Strength Starch, Corn (UNII: O8232NY3SJ) Croscarmellose Sodium (UNII: M28OL1HH48) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) Magnesium Stearate (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) Mineral Oil (UNII: T5L8T28FGP) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) Propylene Glycol (UNII: 6DC9Q167V3) Silicon Dioxide (UNII: ETJ7Z6XBU4) Product Characteristics Color BROWN (Reddish-brown) Score no score Shape ROUND Size 10mm Flavor Imprint Code 702 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 63629-6846-1 30 in 1 BOTTLE; Type 0: Not a Combination Product 11/12/2015 2 NDC: 63629-6846-2 7 in 1 BOTTLE; Type 0: Not a Combination Product 11/12/2015 3 NDC: 63629-6846-3 10 in 1 BOTTLE; Type 0: Not a Combination Product 11/12/2015 4 NDC: 63629-6846-4 14 in 1 BOTTLE; Type 0: Not a Combination Product 11/12/2015 5 NDC: 63629-6846-5 6 in 1 BOTTLE; Type 0: Not a Combination Product 11/12/2015 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date UNAPPROVED DRUG OTHER 08/02/2011 PHENAZOPYRIDINE HYDROCHLORIDE
phenazopyridine hydrochloride tablet, coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 63629-6844(NDC:42937-701) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PHENAZOPYRIDINE HYDROCHLORIDE (UNII: 0EWG668W17) (PHENAZOPYRIDINE - UNII:K2J09EMJ52) PHENAZOPYRIDINE HYDROCHLORIDE 100 mg Inactive Ingredients Ingredient Name Strength Starch, Corn (UNII: O8232NY3SJ) Croscarmellose Sodium (UNII: M28OL1HH48) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) Magnesium Stearate (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) Mineral Oil (UNII: T5L8T28FGP) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) Propylene Glycol (UNII: 6DC9Q167V3) Silicon Dioxide (UNII: ETJ7Z6XBU4) Product Characteristics Color BROWN (Reddish-brown) Score no score Shape ROUND Size 7mm Flavor Imprint Code 701 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 63629-6844-1 30 in 1 BOTTLE; Type 0: Not a Combination Product 11/12/2015 2 NDC: 63629-6844-2 6 in 1 BOTTLE; Type 0: Not a Combination Product 11/12/2015 3 NDC: 63629-6844-3 7 in 1 BOTTLE; Type 0: Not a Combination Product 11/12/2015 4 NDC: 63629-6844-4 10 in 1 BOTTLE; Type 0: Not a Combination Product 11/12/2015 5 NDC: 63629-6844-5 14 in 1 BOTTLE; Type 0: Not a Combination Product 11/12/2015 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date UNAPPROVED DRUG OTHER 08/02/2011 Labeler - Bryant Ranch Prepack (171714327) Establishment Name Address ID/FEI Business Operations Bryant Ranch Prepack 171714327 REPACK(63629-6846, 63629-6844) , RELABEL(63629-6846, 63629-6844)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.

