CICLOPIROX OLAMINE suspension
Ciclopirox Olamine by
Drug Labeling and Warnings
Ciclopirox Olamine by is a Prescription medication manufactured, distributed, or labeled by Bryant Ranch Prepack. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
Ciclopirox Topical Suspension USP, 0.77% (w/w) (Lotion) is for topical use.
Each gram of Ciclopirox Topical Suspension USP, 0.77% (w/w) (Lotion) contains 7.70 mg of ciclopirox (as ciclopirox olamine) in a water miscible suspension base consisting of benzyl alcohol (1% as a preservative), cetyl alcohol, lactic acid, light mineral oil, myristyl alcohol, octyldodecanol, polysorbate 60, purified water, sorbitan monostearate, and stearyl alcohol.
Ciclopirox Topical Suspension USP, 0.77% (w/w) (Lotion) contains a synthetic, broad-spectrum, antifungal agent ciclopirox (as ciclopirox olamine).
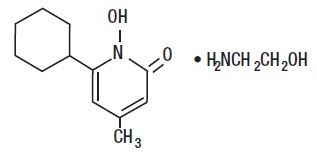
The chemical name is 6-cyclohexyl-1-hydroxy-4-methyl-2(1H)-pyridone, 2-aminoethanol salt.
The CAS Registry Number is 41621-49-2.
Ciclopirox Topical Suspension USP, 0.77% (w/w) (Lotion) has a pH of 7. The chemical structure is:
-
CLINICAL PHARMACOLOGY
Mechanism of Action
Ciclopirox is a hydroxypyridone antifungal agent that acts by chelation of polyvalent cations (Fe3+ or Al3+), resulting in the inhibition of the metal-dependent enzymes that are responsible for the degradation of peroxides within the fungal cell.
Pharmacokinetics
Pharmacokinetic studies in men with radiolabeled ciclopirox solution in polyethylene glycol 400, showed an average of 1.3% absorption of the dose when it was applied topically to 750 cm2 on the back followed by occlusion for 6 hours.
The biological half-life was 1.7 hours and excretion occurred via the kidney. Two days after application only 0.01% of the dose applied could be found in the urine. Fecal excretion was negligible. Autoradiographic studies with human cadaver skin showed that ciclopirox penetrates into the hair and through the epidermis and hair follicles into the sebaceous glands and dermis, while a portion of the drug remains in the stratum corneum.
In vitro penetration studies in frozen or fresh excised human cadaver and pig skin indicated that the penetration of ciclopirox topical suspension, 0.77% is equivalent to that of ciclopirox cream 0.77%. Therapeutic equivalence of cream and suspension formulations was also indicated by studies of experimentally induced guinea pig and human trichophytosis.
-
INDICATIONS AND USAGE
Ciclopirox Topical Suspension USP, 0.77% (w/w) (Lotion) is indicated for the topical treatment of the following dermal infections: tinea pedis, tinea cruris and tinea corporis due to Trichophyton rubrum, Trichophyton mentagrophytes, Epidermophyton floccosum, and Microsporum canis; cutaneous candidiasis (moniliasis) due to Candida albicans; and tinea (pityriasis) versicolor due to Malassezia furfur.
- CONTRAINDICATIONS
- WARNINGS
-
PRECAUTIONS
If a reaction suggesting sensitivity or chemical irritation should occur with the use of Ciclopirox Topical Suspension USP, 0.77% (w/w) (Lotion), treatment should be discontinued and appropriate therapy instituted.
Information for Patients -
The patient should be told to:
- 1. Use the medication for the full treatment time even though signs/symptoms may have improved and notify the physician if there is no improvement after four weeks.
- 2. Inform the physician if the area of application shows signs of increased irritation (redness, itching, burning, blistering, swelling, oozing) indicative of possible sensitization.
- 3. Avoid the use of occlusive wrappings or dressings.
Carcinogenesis, Mutagenesis, Impairment of Fertility -
A 104-week dermal carcinogenicity study in mice was conducted with ciclopirox cream applied at doses up to 1.93% (100 mg/kg/day or 300 mg/m2/day). No increase in drug related neoplasms was noted when compared to control.
The following in vitro genotoxicity tests have been conducted with ciclopirox: evaluation of gene mutation in the Ames Salmonella and E. coli assays (negative); chromosome aberration assays in V79 Chinese hamster lung fibroblast cells, with and without metabolic activation (positive); chromosome aberration assays in V79 Chinese hamster lung fibroblast cells in the presence of supplemental Fe3+, with and without metabolic activation (negative); gene mutation assays in the HGPRT test with V79 Chinese hamster lung fibroblast cells (negative); and a primary DNA damage assay (i.e., unscheduled DNA synthesis assay in A549 human celIs) (negative). An in vitro cell transformation assay in BALB/c 3T3 cells was negative for cell transformation. In an in vivo Chinese hamster bone marrow cytogenetic assay, ciclopirox was negative for chromosome aberrations at a dosage of 5000 mg/kg body weight.
A combined oral fertility and embryofetal developmental study was conducted in rats with ciclopirox olamine. No effect on fertility or reproductive performance was noted at the highest dose tested of 3.85 mg/kg/day ciclopirox (approximately 1.2 times the maximum recommended human dose based on body surface area comparisons).
Pregnancy
Teratogenic Effects: Pregnancy Category B
There are no adequate or well-controlled studies in pregnant women. Therefore, Ciclopirox Topical Suspension USP, 0.77% (w/w) (Lotion) should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Oral embryofetal developmental studies were conducted in mice, rats, rabbits and monkeys. Ciclopirox or ciclopirox olamine was orally administered during the period of organogenesis. No maternal toxicity, embryotoxicity or teratogenicity were noted at the highest doses of 77, 125, 80 and 38.5 mg/kg/day ciclopirox in mice, rats, rabbits and monkeys, respectively (approximately 11, 37, 51 and 24 times the maximum recommended human dose based on body surface area comparisons, respectively).
Dermal embryofetaI developmental studies were conducted in rats and rabbits with ciclopirox olamine dissolved in PEG 400. Ciclopirox olamine was topically administered during the period of organogenesis. No maternal toxicity, embryotoxicity or teratogenicity were noted at the highest doses of 92 mg/kg/day and 77 mg/kg/day ciclopirox in rats and rabbits, respectively (approximately 27 and 49 times the maximum recommended human dose based on body surface area comparisons, respectively).
-
ADVERSE REACTIONS
In the controlled clinical trial with 89 patients using ciclopirox topical suspension and 89 patients using the vehicle, the incidence of adverse reactions was low. Those considered possibly related to treatment or occurring in more than one patient were pruritus, which occurred in two patients using ciclopirox topical suspension and one patient using the suspension vehicle, and burning, which occurred in one patient using ciclopirox topical suspension.
-
DOSAGE AND ADMINISTRATION
Gently massage Ciclopirox Topical Suspension USP, 0.77% (w/w) (Lotion) into the affected and surrounding skin areas twice daily, in the morning and evening. Clinical improvement with relief of pruritus and other symptoms usually occurs within the first week of treatment. If a patient shows no clinical improvement after four weeks of treatment with Ciclopirox Topical Suspension USP, 0.77% (w/w) (Lotion) the diagnosis should be redetermined. Patients with tinea versicolor usually exhibit clinical and mycological clearing after two weeks of treatment.
-
HOW SUPPLIED
Ciclopirox Olamine Topical Suspension USP, 0.77% (w/w) (Lotion) is available as follows:
- 30 mL bottle (NDC: 63629-2522-1)
Bottle space provided to allow for vigorous shaking before each use.
Repackaged/Relabeled by:
Bryant Ranch Prepack, Inc.
Burbank, CA 91504 - STORAGE AND HANDLING
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CICLOPIROX OLAMINE
ciclopirox olamine suspensionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 63629-2522(NDC:45802-400) Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CICLOPIROX OLAMINE (UNII: 50MD4SB4AP) (CICLOPIROX - UNII:19W019ZDRJ) CICLOPIROX 7.70 mg in 100 mL Inactive Ingredients Ingredient Name Strength BENZYL ALCOHOL (UNII: LKG8494WBH) CETYL ALCOHOL (UNII: 936JST6JCN) LACTIC ACID, UNSPECIFIED FORM (UNII: 33X04XA5AT) LIGHT MINERAL OIL (UNII: N6K5787QVP) MYRISTYL ALCOHOL (UNII: V42034O9PU) OCTYLDODECANOL (UNII: 461N1O614Y) POLYSORBATE 60 (UNII: CAL22UVI4M) WATER (UNII: 059QF0KO0R) SORBITAN MONOSTEARATE (UNII: NVZ4I0H58X) STEARYL ALCOHOL (UNII: 2KR89I4H1Y) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 63629-2522-1 1 in 1 CARTON 12/29/2006 1 30 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA077676 12/29/2006 Labeler - Bryant Ranch Prepack (171714327) Registrant - Bryant Ranch Prepack (171714327) Establishment Name Address ID/FEI Business Operations Bryant Ranch Prepack 171714327 REPACK(63629-2522) , RELABEL(63629-2522)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.