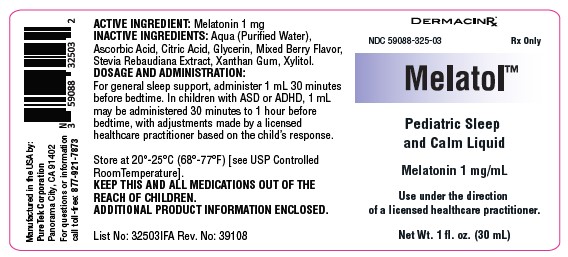
MELATOL- melatonin liquid
Melatol by
Drug Labeling and Warnings
Melatol by is a Prescription medication manufactured, distributed, or labeled by PureTek Corporation. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
DESCRIPTION
Melatonin is a naturally occurring hormone produced by the pineal gland in the brain that helps regulate the sleep-wake cycle. In children, melatonin is commonly used as a supplement to support healthy sleep patterns, particularly in those with conditions like Autism Spectrum Disorder (ASD) and Attention-Deficit/Hyperactivity Disorder (ADHD).
-
CLINICAL PHARMACOLOGY
Melatonin plays a crucial role in regulating circadian rhythms, including the sleep-wake cycle. Its secretion is stimulated by darkness and inhibited by light, helping to establish and maintain the body’s internal clock. In children, melatonin supplementation can be beneficial for those with disrupted sleep patterns due to conditions like ASD and ADHD, promoting sleep onset and improving sleep quality.
- Pharmacodynamics:
-
Pharmacokinetics:
After oral administration, melatonin is rapidly absorbed, with peak plasma concentrations reached within 20 to 60 minutes. The bioavailability of melatonin is generally around 15%. It is metabolized primarily in the liver by the cytochrome P450 enzyme system, particularly CYP1A2, and the major metabolite, 6-sulfatoxymelatonin, is excreted in the urine. The half-life of melatonin in plasma ranges from 30 to 60 minutes. Pharmacokinetics in children may vary based on age, weight, and metabolic rate, with melatonin best administered shortly before bedtime to maximize its sleep-promoting effects.
-
INDICATIONS AND USAGE
Melatol™ Pediatric Sleep and Calm Liquid is intended for occasional or intermittent use to manage sleep-related issues in pediatric patients. It may be recommended for children with sleep disorders such as insomnia, ASD, or ADHD to help regulate sleep-wake cycles, improve sleep onset, and enhance overall sleep quality. This product is intended for children aged 3 years and older and should be used under the guidance of a licensed healthcare practitioner.
-
CONTRAINDICATIONS
Melatol™ Pediatric Sleep and Calm Liquid is contraindicated in children with hypersensitivity or allergic reactions to melatonin or any of the ingredients in the formulation. It should not be used in children with known autoimmune disorders, as melatonin may stimulate the immune system and exacerbate these conditions. Additionally, it is contraindicated in children with severe hepatic impairment due to altered melatonin metabolism and in those receiving immunosuppressive therapy, as melatonin may reduce the effectiveness of these treatments. Melatonin use is also contraindicated during pregnancy and lactation due to insufficient safety data.
-
WARNINGS AND PRECAUTIONS
Consult a licensed healthcare practitioner before starting melatonin, especially if the child has a medical condition or is taking other medications. Melatonin may cause daytime drowsiness, so ensure that the child avoids activities requiring alertness, such as riding a bike or climbing, after taking melatonin. Discontinue use and seek medical advice if any adverse reactions occur.
-
ADVERSE REACTIONS
Possible adverse reactions include daytime drowsiness, headaches, dizziness, gastrointestinal issues such as nausea or stomach cramps, and mood changes like irritability or agitation. Allergic reactions, although rare, may include rash, itching, swelling, or difficulty breathing. The potential impact on hormonal development, particularly puberty, is not well understood, so long-term use should be monitored.
-
DRUG INTERACTIONS
Melatol™ Pediatric Sleep and Calm Liquid may enhance the effects of sedatives, hypnotics, and antidepressants, leading to increased drowsiness. It may also interact with medications metabolized by the liver, potentially altering their efficacy. Use with immunosuppressive drugs may reduce their effectiveness, and there is a potential increased risk of bleeding when taken with anticoagulants. Consult a licensed healthcare practitioner before administering melatonin if the child is taking other medications or supplements.
-
USE IN SPECIFIC POPULATIONS
Pediatric Use: Melatol™ Pediatric Sleep and Calm Liquid is specifically formulated for pediatric patients and should be used under the guidance of a licensed healthcare practitioner. The safety and efficacy of melatonin for long-term use or in high doses for children have not been fully established.
Geriatric Use: This formulation is intended for pediatric use, and data on its use in elderly patients are not applicable.
Hepatic Impairment: Use in children with severe hepatic impairment is not recommended due to altered melatonin metabolism and increased risk of adverse effects.
Renal Impairment: There is limited data on melatonin use in children with renal impairment. Caution is advised, and a licensed healthcare practitioner should assess the risk-benefit ratio before use.
-
OVERDOSAGE
In case of overdose, symptoms may include excessive drowsiness, headache, dizziness, nausea, and irritability. In rare cases, more severe symptoms such as confusion, disorientation, or low blood pressure may occur. Treatment should be symptomatic and supportive. If overdose is suspected, contact a poison control center or seek immediate medical attention from a licensed healthcare provider.
-
NONCLINICAL TOXICOLOGY
Carcinogenesis, Mutagenesis, Impairment of Fertility: Nonclinical studies have shown no evidence of carcinogenicity, mutagenicity, or impairment of fertility at doses significantly higher than those used in humans. However, the long-term effects of melatonin on growth, development, and reproductive health in pediatric populations have not been fully established.
Reproductive and Developmental Toxicology: Animal studies have not shown adverse effects on reproductive or developmental outcomes at doses comparable to those used in pediatric patients. However, since these studies may not predict human response, melatonin should only be used in children when clearly needed and under supervision.
- DOSAGE AND ADMINISTRATION
-
HOW SUPPLIED
Melatol™ Pediatric Sleep and Calm Liquid is supplied in a 1 fl. oz. (30 mL) bottle (NDC: 59088-325-03).
KEEP THIS AND ALL MEDICATIONS OUT OF THE REACH OF CHILDREN. Store at 20°-25°C (68°-77°F) [see USP Controlled Room Temperature].
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
MELATOL
melatonin liquidProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 59088-325 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MELATONIN (UNII: JL5DK93RCL) (MELATONIN - UNII:JL5DK93RCL) MELATONIN 1 mg in 30 mL Inactive Ingredients Ingredient Name Strength XYLITOL (UNII: VCQ006KQ1E) WATER (UNII: 059QF0KO0R) XANTHAN GUM (UNII: TTV12P4NEE) REBAUDIOSIDE A (UNII: B3FUD0528F) ASCORBIC ACID (UNII: PQ6CK8PD0R) GLYCERIN (UNII: PDC6A3C0OX) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 59088-325-03 30 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product 10/17/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 10/17/2024 Labeler - PureTek Corporation (785961046)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.