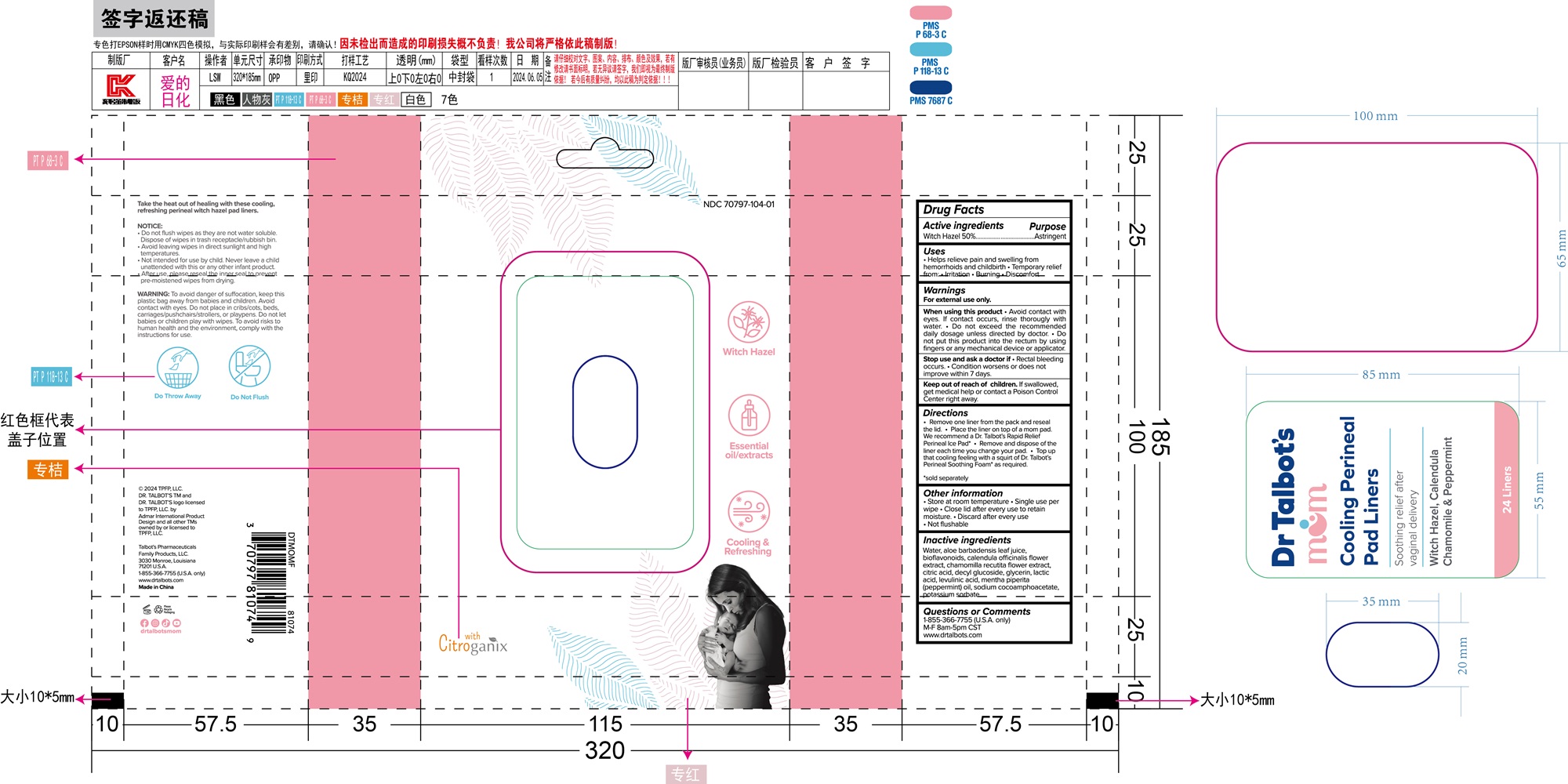
Cooling Perineal Pad Liners by Ningbo Riway Daily Commodity Co., Ltd / Ningbo Riway Industrial Co., Ltd
Cooling Perineal Pad Liners by
Drug Labeling and Warnings
Cooling Perineal Pad Liners by is a Otc medication manufactured, distributed, or labeled by Ningbo Riway Daily Commodity Co., Ltd, Ningbo Riway Industrial Co., Ltd. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
COOLING PERINEAL PAD LINERS- cooling perineal cloth
Ningbo Riway Daily Commodity Co., Ltd
----------
* Help relieve pain and swelling from hemorrhoids and childbirth
* Temporary relief from: irritation, burning, discomfort
When using this product:
* Avoid contact with eyes. If contact occurs, rinse thorougly with water.
* Do not exceed the recommended daily dosage unless directed by doctor.
* Do not put this product into the rectum by using fingers or any mechanical device or applicator.
Stop use and ask and ask a dotor if
* Rectal bleeding occurs.
* Condition worsens or does not improve within 7 days.
* Remove one liner from the pack and reseal the lid.
* Place the liner on top of a mom pad.
* Remove and dispose of the liner each time you change your pad.
* Top up that cooling feeling with a squirt of Dr. Talbot's Perineal Soothing Foam as required.
Water, aloe barbadensis leaf juice, boiflavonoids, calendula offcinalis flower extract, chamomilla recutita flower extract, citric acid, decyl glucoside, glycerin, lactic acid, levulinic acid, mentha piperita oil, sodium cocoamphoacetate, potassium sorbate
* Store at room temperature
* Single use per wipe
* Close lid after every use to retain moisture
* Discard after every use
* Not flushable
| COOLING PERINEAL PAD LINERS
cooling perineal cloth |
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
| Labeler - Ningbo Riway Daily Commodity Co., Ltd (540997562) |
| Registrant - Ningbo Riway Industrial Co., Ltd (540997562) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Ningbo Riway Daily Commodity Co., Ltd | 540997562 | manufacture(77267-016) | |