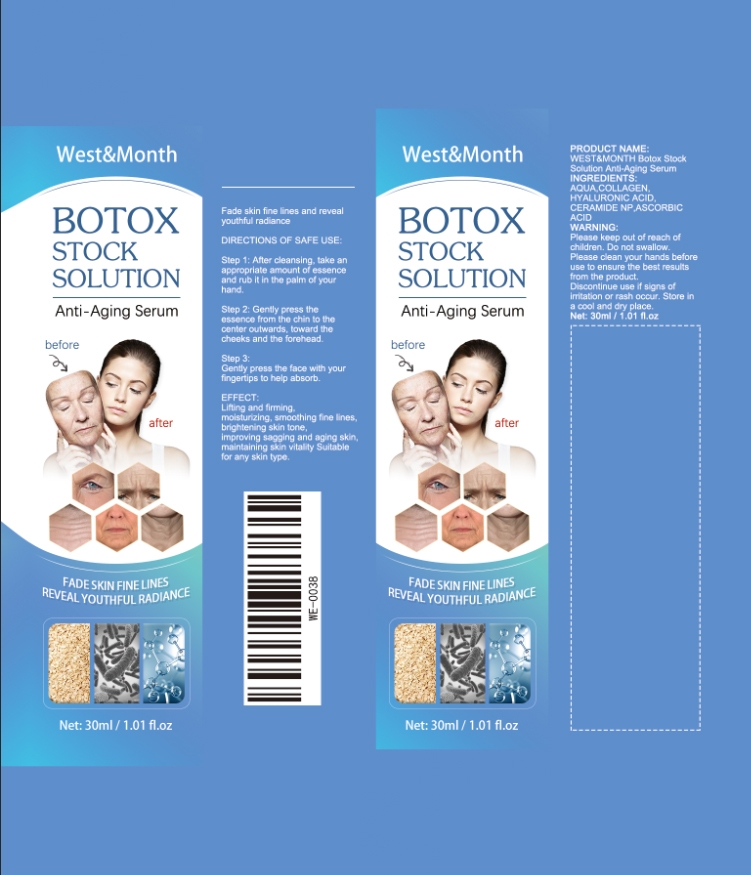
Botox Stock Solution Anti-Aging Serum by Shenzhenshishengyakeji Co., Ltd.
Botox Stock Solution Anti-Aging Serum by
Drug Labeling and Warnings
Botox Stock Solution Anti-Aging Serum by is a Otc medication manufactured, distributed, or labeled by Shenzhenshishengyakeji Co., Ltd.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
BOTOX STOCK SOLUTION ANTI-AGING SERUM- botox stock solution anti-aging serum liquid
Shenzhenshishengyakeji Co., Ltd.
----------
1.Step 1: After cleansing, take an
appropriate amount of essence
and rub it in the palm of your
hand.
2.Genty press the essence from the chin to the center outwards, toward the cheeks and the forehead.
3.Gently press the face with your fingertps to help absorb.
| BOTOX STOCK SOLUTION ANTI-AGING SERUM
botox stock solution anti-aging serum liquid |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Shenzhenshishengyakeji Co., Ltd. (619715687) |
| Registrant - Shenzhenshishengyakeji Co., Ltd. (619715687) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Shenzhenshishengyakeji Co., Ltd. | 619715687 | label(84752-004) | |
Revised: 10/2024
Document Id: 24956b63-df8d-ded6-e063-6294a90ad880
Set id: 24956e23-f7d9-7822-e063-6394a90a0ecd
Version: 1
Effective Time: 20241012