Bittol Anti-Bacterial Wet Wipes
Bittol Anti-Bacterial Wet Wipes by
Drug Labeling and Warnings
Bittol Anti-Bacterial Wet Wipes by is a Otc medication manufactured, distributed, or labeled by Taha Kimya Kozmetik Ve Tuketim Urunleri Sanayi Pazarlama Limited Sirketi. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
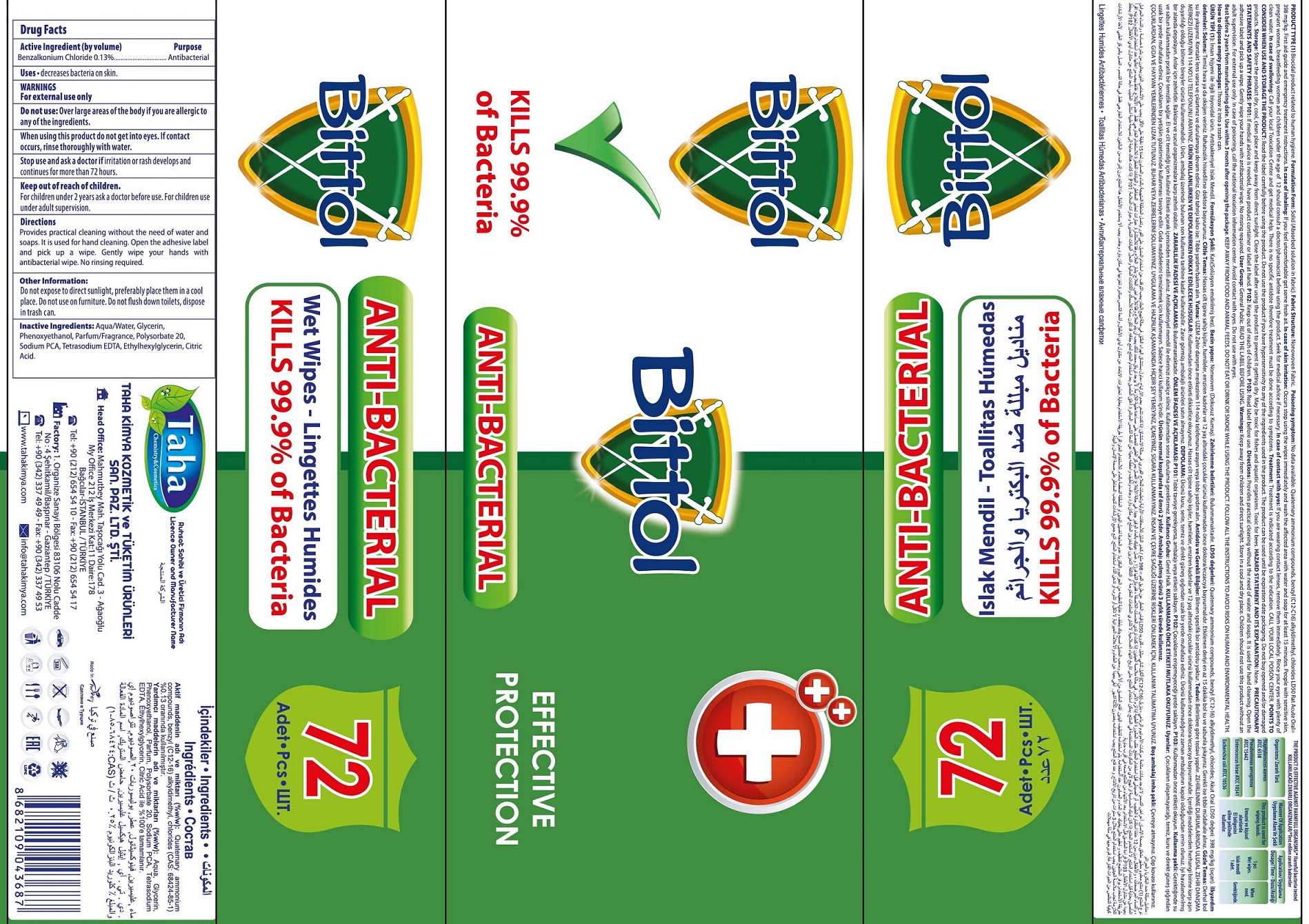
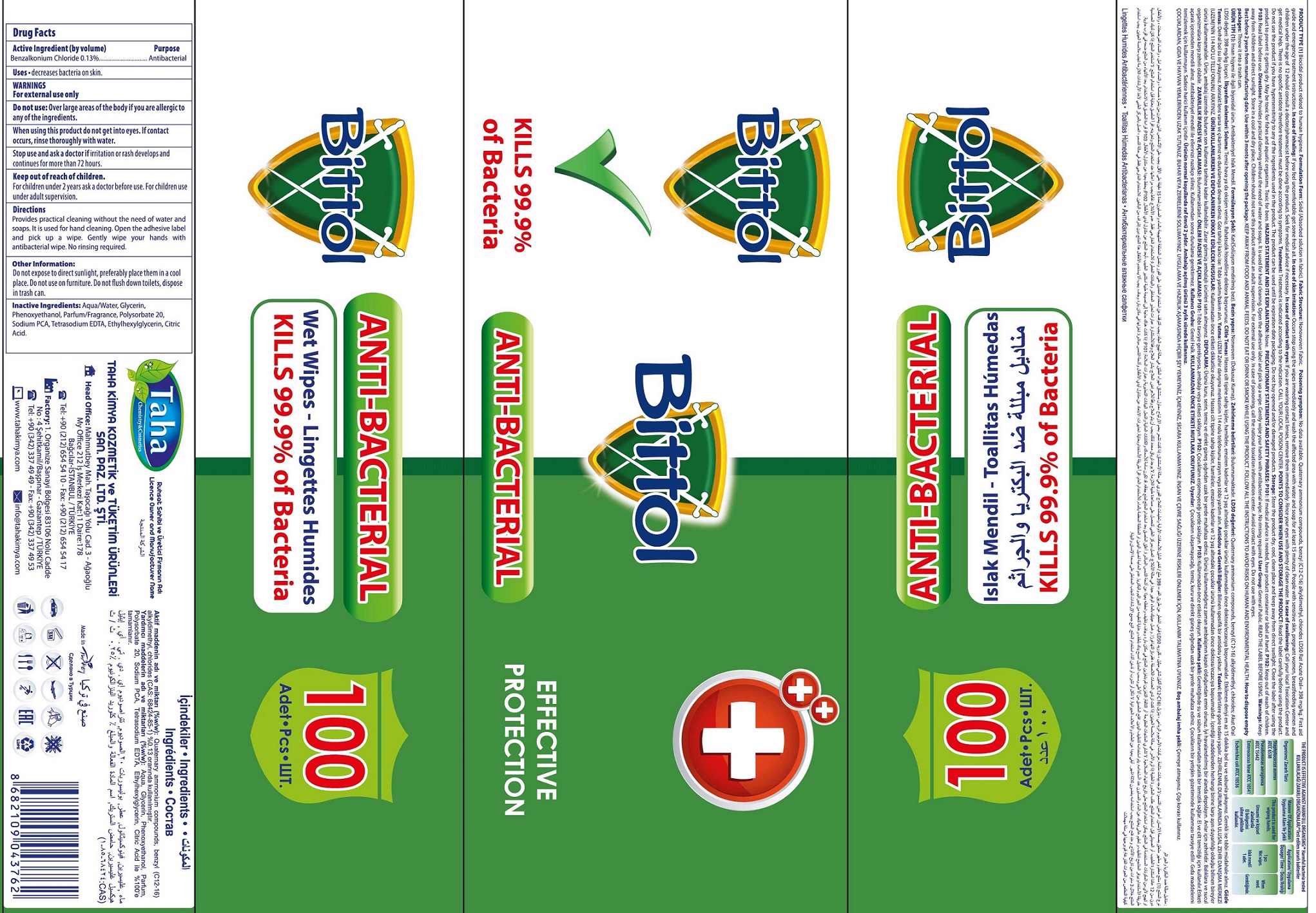
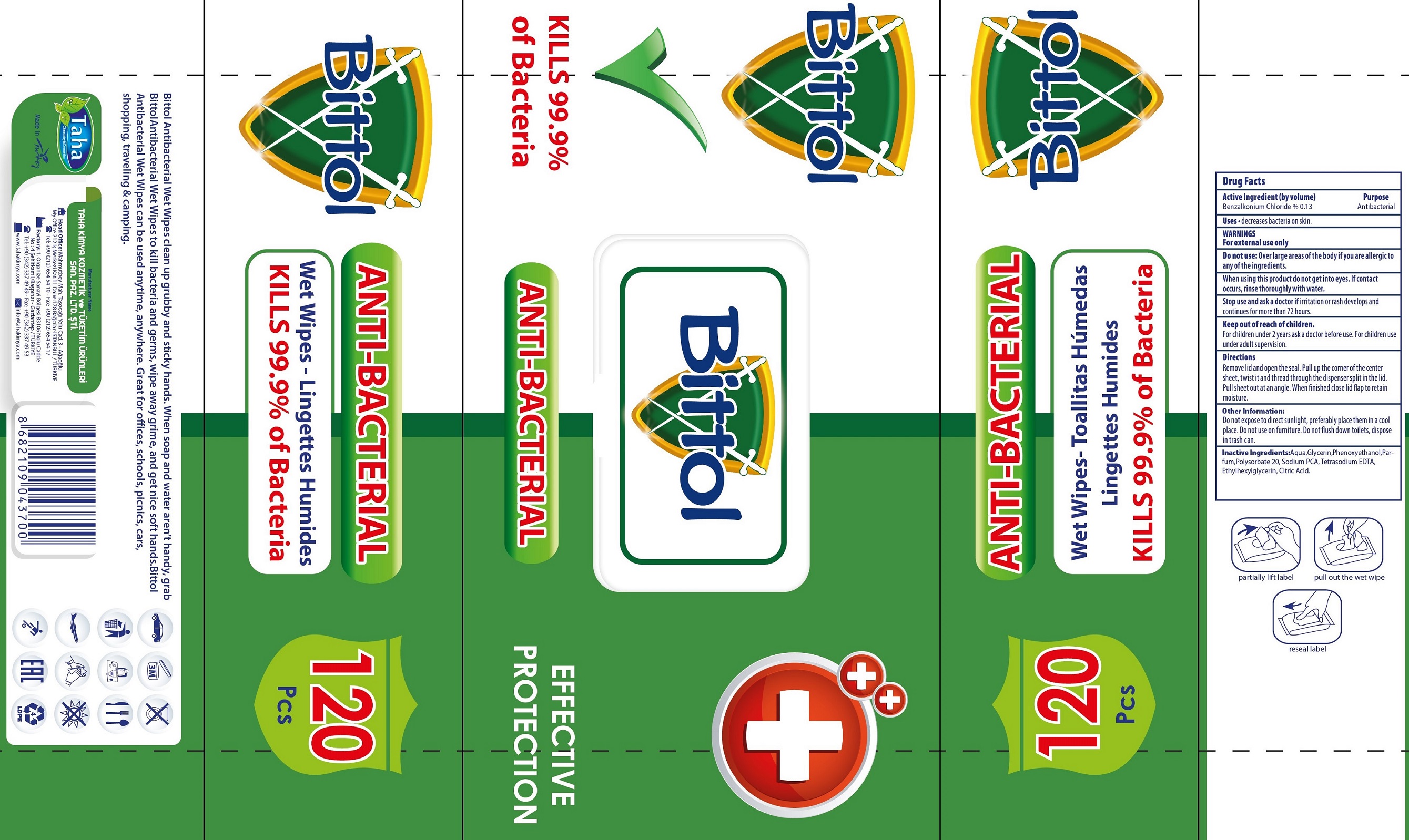
BITTOL ANTI-BACTERIAL WET WIPES- benzalkonium chloride cloth
Taha Kimya Kozmetik Ve Tuketim Urunleri Sanayi Pazarlama Limited Sirketi
----------
Bittol Anti-Bacterial Wet Wipes
Directions
Removelid and open the seal. Pull up the corner of the center sheet, twist and thread through the dispenser split in the lid. Pull sheet out at an angle. When finished close lid flap to retain moisture.
Other Information:
Do not expose to direct sunlight, preferably place them in a cool place. Do not use on furniture. Do not flush down toilets, dispose in trash can.
| BITTOL ANTI-BACTERIAL WET WIPES
benzalkonium chloride cloth |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Taha Kimya Kozmetik Ve Tuketim Urunleri Sanayi Pazarlama Limited Sirketi (565657236) |
Revised: 5/2024
Document Id: 19c34213-51e2-79a8-e063-6394a90a92bd
Set id: 24d91fdf-3b2e-4c9c-b0a8-c6f593476e0e
Version: 4
Effective Time: 20240531
Taha Kimya Kozmetik Ve Tuketim U
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.