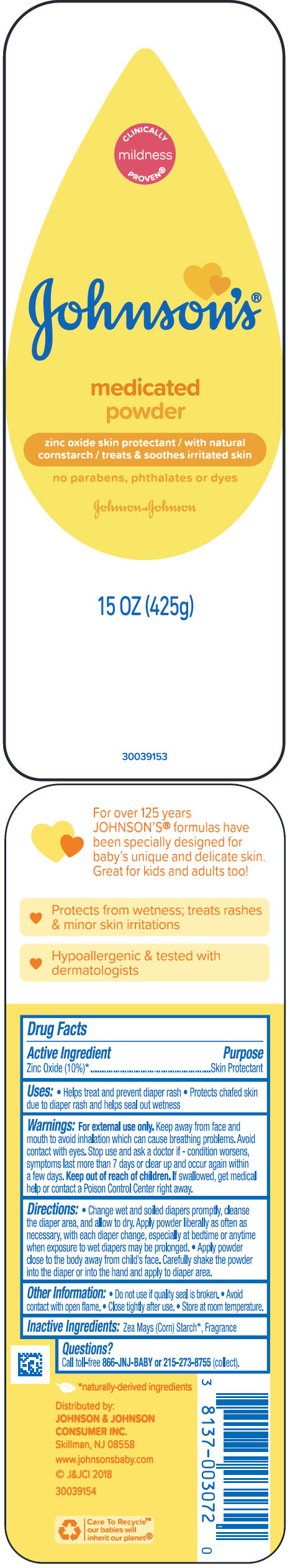
JOHNSONS MEDICATED- zinc oxide powder
Johnsons medicated by
Drug Labeling and Warnings
Johnsons medicated by is a Otc medication manufactured, distributed, or labeled by Johnson & Johnson Consumer Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
-
Active ingredient
Zinc Oxide (10%)1
- Purpose
- Uses
- Warnings
-
Directions
- Change wet and soiled diapers promptly, cleanse the diaper area, and allow to dry. Apply powder liberally as often as necessary, with each diaper change, especially at bedtime or anytime when exposure to wet diapers may be prolonged.
- Apply powder close to the body away from child's face. Carefully shake the powder into the diaper or into the hand and apply to diaper area.
- Other information
- Inactive ingredients
- Questions ?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 425 g Bottle Label
-
INGREDIENTS AND APPEARANCE
JOHNSONS MEDICATED
zinc oxide powderProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 69968-0493 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Zinc oxide (UNII: SOI2LOH54Z) (Zinc oxide - UNII:SOI2LOH54Z) Zinc oxide 100 mg in 1 g Inactive Ingredients Ingredient Name Strength STARCH, CORN (UNII: O8232NY3SJ) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 69968-0493-1 425 g in 1 BOTTLE; Type 0: Not a Combination Product 06/01/2005 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part347 06/01/2005 Labeler - Johnson & Johnson Consumer Inc. (002347102)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.