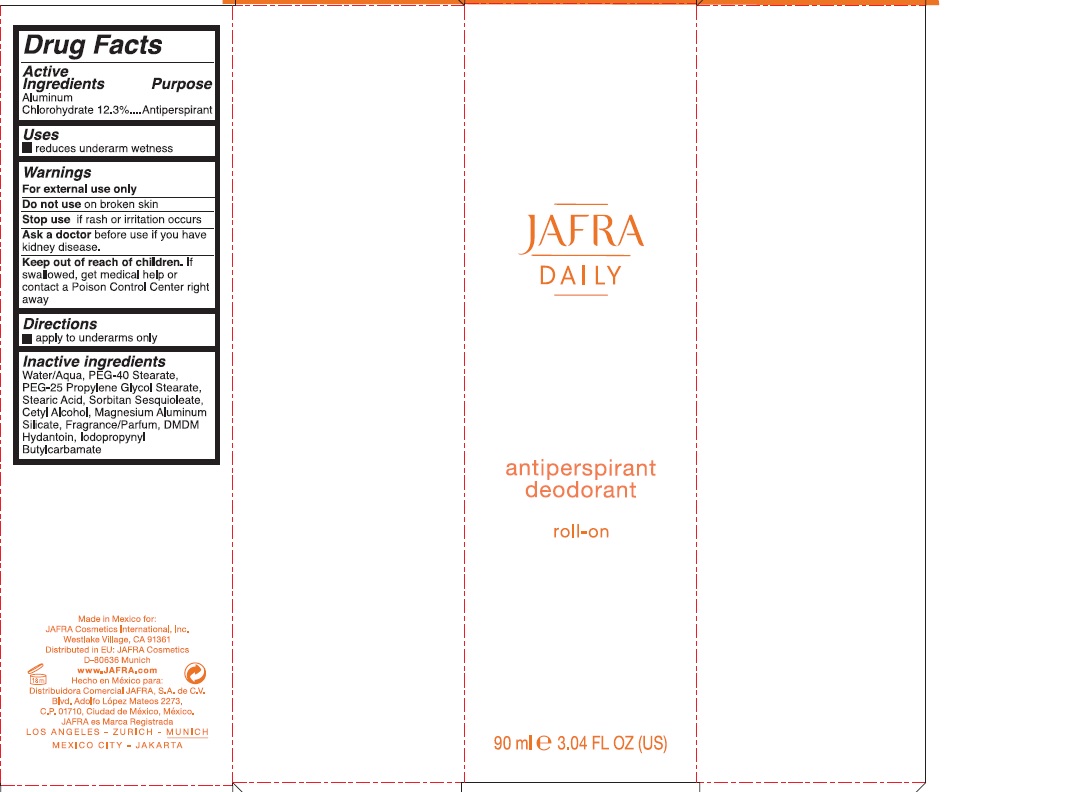
Jafra Daily Antiperspirant Rollon by Jafra Cosmetics International Inc / Distribuidora Comercial Jafra, S.A. de C.V.
Jafra Daily Antiperspirant Rollon by
Drug Labeling and Warnings
Jafra Daily Antiperspirant Rollon by is a Otc medication manufactured, distributed, or labeled by Jafra Cosmetics International Inc, Distribuidora Comercial Jafra, S.A. de C.V.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
JAFRA DAILY ANTIPERSPIRANT ROLLON- aluminum chlorohydrate liquid
Jafra Cosmetics International Inc
----------
Warnings
For external use only
Do not useon broken skin
Stop useif rash or irritation occurs
Ask a doctorbefore use if you have kidney disease
Keep out of reach of children.If swallowed, get medical help or contact a Poison Control Center right away
| JAFRA DAILY ANTIPERSPIRANT ROLLON
aluminum chlorohydrate liquid |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - Jafra Cosmetics International Inc (041676479) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Distribuidora Comercial Jafra, S.A. de C.V. | 951612777 | manufacture(68828-503) | |
Revised: 1/2024
Document Id: 0e37cf4e-6e7c-841b-e063-6394a90ad008
Set id: 25447aa1-917b-48cd-8920-94d510d14e6d
Version: 2
Effective Time: 20240105