Ultra Premium Compound Essential Oil by Shenzhenshishengyakeji Co., Ltd.
Ultra Premium Compound Essential Oil by
Drug Labeling and Warnings
Ultra Premium Compound Essential Oil by is a Otc medication manufactured, distributed, or labeled by Shenzhenshishengyakeji Co., Ltd.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
ULTRA PREMIUM COMPOUND ESSENTIAL OIL- ultra premium compound essential oil liquid
Shenzhenshishengyakeji Co., Ltd.
----------
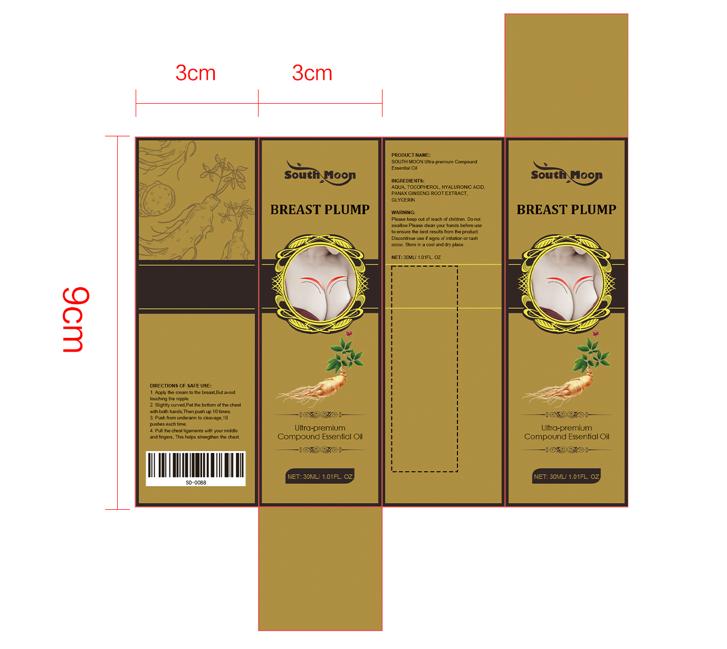
1.Apply the cream to the breast But avoid touching the nipple.
2.Stightly curved Pat the bottom of the chest with both hands,
Then push up 10 times.
3.Push from underarm to cleavage,10 pushes each time.
4. Pull the chest ligaments with your middle and fingers,
This helps strengthen the chest.
| ULTRA PREMIUM COMPOUND ESSENTIAL OIL
ultra premium compound essential oil liquid |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Shenzhenshishengyakeji Co., Ltd. (619715687) |
| Registrant - Shenzhenshishengyakeji Co., Ltd. (619715687) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Shenzhenshishengyakeji Co., Ltd. | 619715687 | label(84752-019) | |
Revised: 10/2024
Document Id: 257189e7-1058-3ae7-e063-6294a90a99cb
Set id: 257189e7-1057-3ae7-e063-6294a90a99cb
Version: 1
Effective Time: 20241027