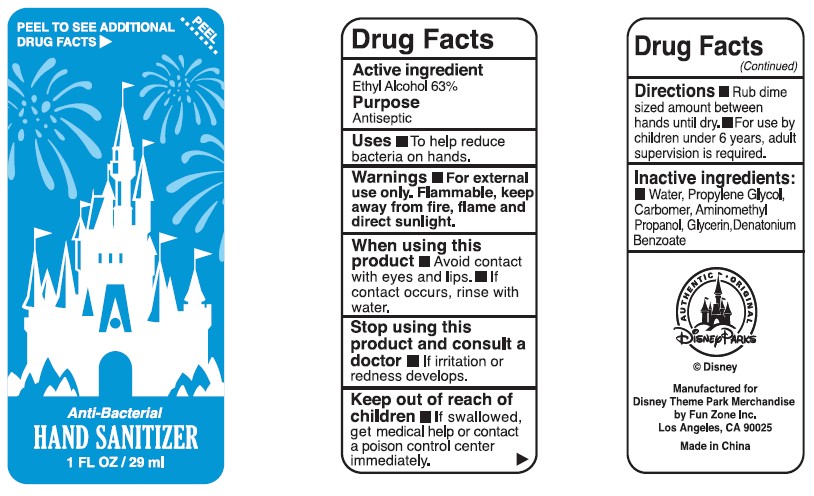
Anti-Bacterial Hand Sanitizer by Fun Zone, Inc.
Anti-Bacterial Hand Sanitizer by
Drug Labeling and Warnings
Anti-Bacterial Hand Sanitizer by is a Otc medication manufactured, distributed, or labeled by Fun Zone, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
ANTI-BACTERIAL HAND SANITIZER- ethyl alcohol gel
Fun Zone, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Stop using this product and consult a doctor If irritation or redness develops.Stop using this product and consult a doctor If irritation or redness develops.
Directions
- Rub dime sized amount between hands until dry.
- For use by children under 6 years, adult supervision is required.
| ANTI-BACTERIAL HAND SANITIZER
ethyl alcohol gel |
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
| Labeler - Fun Zone, Inc. (803572929) |
Revised: 2/2020
Document Id: 9e638fe9-107a-a454-e053-2995a90ae9b6
Set id: 25733a96-bfed-106d-e054-00144ff88e88
Version: 2
Effective Time: 20200212
Fun Zone, Inc.