Amorophen hydrochloride liniment
Amorophen hydrochloride liniment by
Drug Labeling and Warnings
Amorophen hydrochloride liniment by is a Otc medication manufactured, distributed, or labeled by ZheJiang Longmed Medical Technology Co., Ltd.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
AMOROPHEN HYDROCHLORIDE LINIMENT- amorolfine hydrochloride liquid
ZheJiang Longmed Medical Technology Co., Ltd.
----------
Amorophen hydrochloride liniment
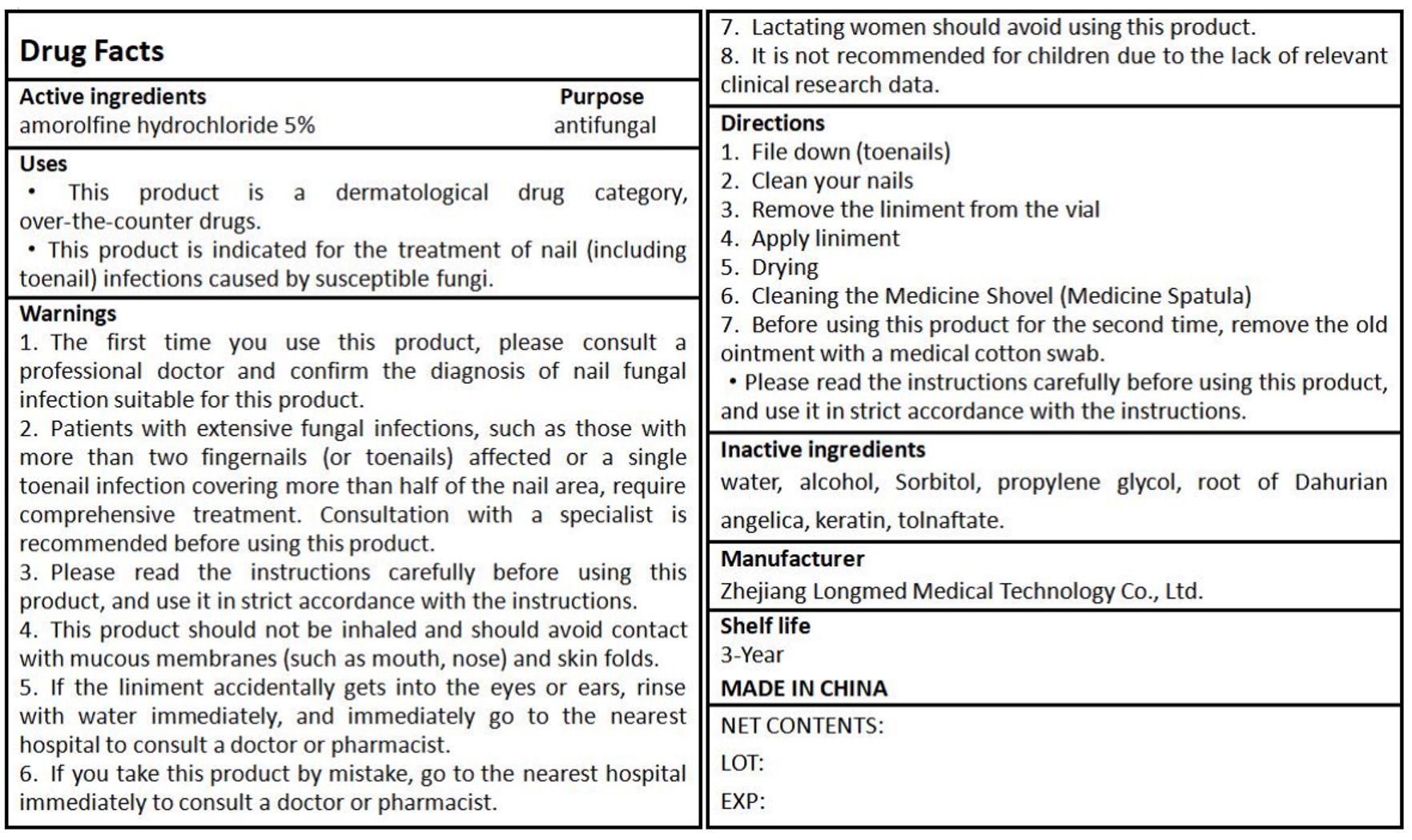
Thisproduct isdermatological drugcategory,over-the-counter drugs.
This product is indicated for the treatment of nail (includingtoenail) infections caused by susceptible fungi.
1. The first time you use this product, please consult aprofessional doctor and confirm the diagnosis of nail fungalinfection suitable for this product.
2. Patients with extensive fungal infections, such as those withmore than two fingernails (or toenails) affected or a singletoenail infection covering more than half of the nail area, requirecomprehensive treatment. Consultation with a specialist isrecommended before using this product.
3.Please read the instructions carefully before usingthisproduct, and use it in strict accordance with the instructions.
4. This product should not be inhaled and should avoid contactwith mucous membranes (such as mouth, nose) and skin folds.
5. lf the liniment accidentally gets into the eyes or ears, rinsewith water immediately, and immediately go to the nearesthospital to consult a doctor or pharmacist.
6. lf you take this product by mistake, go to the nearest hospitalimmediately to consult a doctor or pharmacist.
7. Lactating women should avoid using this product.
8. lt is not recommended for children due to the lack of relevantclinical research data.
1. File down (toenails)
2. Clean your nails
3. Remove the liniment from the vial4. Apply liniment
5. Drying
6. Cleaning the Medicine Shovel (Medicine spatula)
7. Before using this product for the second time, remove the oldointment with a medical cotton swab.
Please read the instructions carefully before using this product,and use it in strict accordance with the instructions.
| AMOROPHEN HYDROCHLORIDE LINIMENT
amorolfine hydrochloride liquid |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Labeler - ZheJiang Longmed Medical Technology Co., Ltd. (554468373) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| ZheJiang Longmed Medical Technology Co., Ltd. | 554468373 | manufacture(84534-002) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.