Sodium Chloride by Global Guard Services LLC / Shijiazhuang No 4 Pharmaceutical Co Ltd
Sodium Chloride by
Drug Labeling and Warnings
Sodium Chloride by is a Prescription medication manufactured, distributed, or labeled by Global Guard Services LLC, Shijiazhuang No 4 Pharmaceutical Co Ltd. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
SODIUM CHLORIDE- sodium chloride injection, solution
Global Guard Services LLC
----------
Sodium Chloride IFU
 0.9% Sodium Chloride Injection
0.9% Sodium Chloride Injection
This product is an isotonic sterilizing aqueous solution of sodium chloride.
Chemical Name: Sodium chloride
Molecular Formula: NaCl
Molecular Weight: 58.44
Osmolality: 260~320 mOsmol/kg.
[Character] This product is colorless and clear liquid.
[Indications]
It is used to regulate the balance of water and electrolytes in the body.
[Specification]
50ml: 0.45g; 100ml: 0.9g; 250ml: 2.25g; 500ml: 4.5g; 1000ml: 9g
[Dosage and Usage]
Intravenous infusion, dosage according to the need of the disease.
[Adverse Reactions]
Excessive and rapid infusion can cause water and sodium retention, edema, elevated blood
pressure, rapid heart rate, chest tightness, dyspnea, and even acute left heart failure.
[Precautions]
(1) Use with caution in the following cases:
① Edematous diseases, such as nephrotic syndrome, cirrhosis ascites, congestive heart
failure, acute left heart failure, brain edema and idiopathic edema;
② Acute renal failure oliguria stage, chronic renal failure urine volume decreased and
poor response to diuretic drugs;
③ Hypertension;
④ Hypokalemia.
(2) Follow-up examination: ① Serum sodium, potassium, chlorine concentration; ② Blood
acid-base balance index; ③ Kidney function; ④ Blood pressure and cardiopulmonary
function.
[Medication in pregnant women and lactating women]
Not clear.
[Medication in children]
The amount and speed of fluid supplementation should be strictly controlled.
[Medication in the elderly]
The amount and speed of fluid supplementation should be strictly controlled.
[Drug interactions]
Should not be used with drugs with known incompatibility contraindications.
[Drug overdose]
For those with existing acidosis, a large amount of this product can cause hyperchloric
acidosis.
[Pharmacology and toxicology]
Sodium and chlorine are important electrolytes in the body, mainly in the extracellular fluid,
and play a very important role in maintaining the normal blood and extracellular fluid
volume and osmotic pressure of the human body. The normal serum sodium concentration is
135~145mmol/L, accounting for 92% of the plasma cation and 90% of the total osmotic
pressure, so the amount of plasma sodium plays a decisive role in the osmotic pressure. The
normal serum chlorine concentration is 98~106mmol/L, which is mainly regulated by the
human body through the hypothalamus, posterior pituitary and kidney to maintain the
stability of body fluid volume and osmotic pressure.
[Pharmacokinetics]
In the gastrointestinal tract, sodium is absorbed almost entirely through the active transport
of intestinal mucosal cells. Sodium is excreted primarily by the kidneys.
[Storage] Sealed storage.
[Package]
1. Plastic infusion bag (non-PVC co-extruded film, double hard tube, easy to fold, double
valve and double plug). 50ml/ bag, 100ml/ bag, 250ml/ bag, 500ml/ bag, 1000ml/ bag.
2. Plastic infusion bag (non-PVC co-extruded film, double hard tube, easy to fold, double
valve double plug, double aseptic packaging). 50ml/ bag, 100ml/ bag, 250ml/ bag, 500ml/
bag, 1000ml/ bag.
[Validity]
(1) The product of 50ml,100ml and 1000ml specifications is valid for 24 months.
(2) The validity period of 250ml,500ml is 36 months.
[Executive standard] Pharmacopoeia of the People's Republic of China (2020, Volume II)
[Pharmaceutical marketing authorization holder]
Name: Huaren Pharmaceutical Co., Ltd.
Registered Address: No. 187, Zhuzhou Road, Qingdao High-tech Park, China.
[Manufacturer]
Enterprise Name: Shijiazhuang No 4 Pharmaceutical Co., Ltd.
Production Address: No. 518, East Huaian Road, High-tech Industrial Development Zone, Shijiazhuang, China.
Zip code: 050035
Tel.: 0086-0311-67167144
Website:http//www.sjzsiyao.com
Sodium Chloride Bag Label Printing
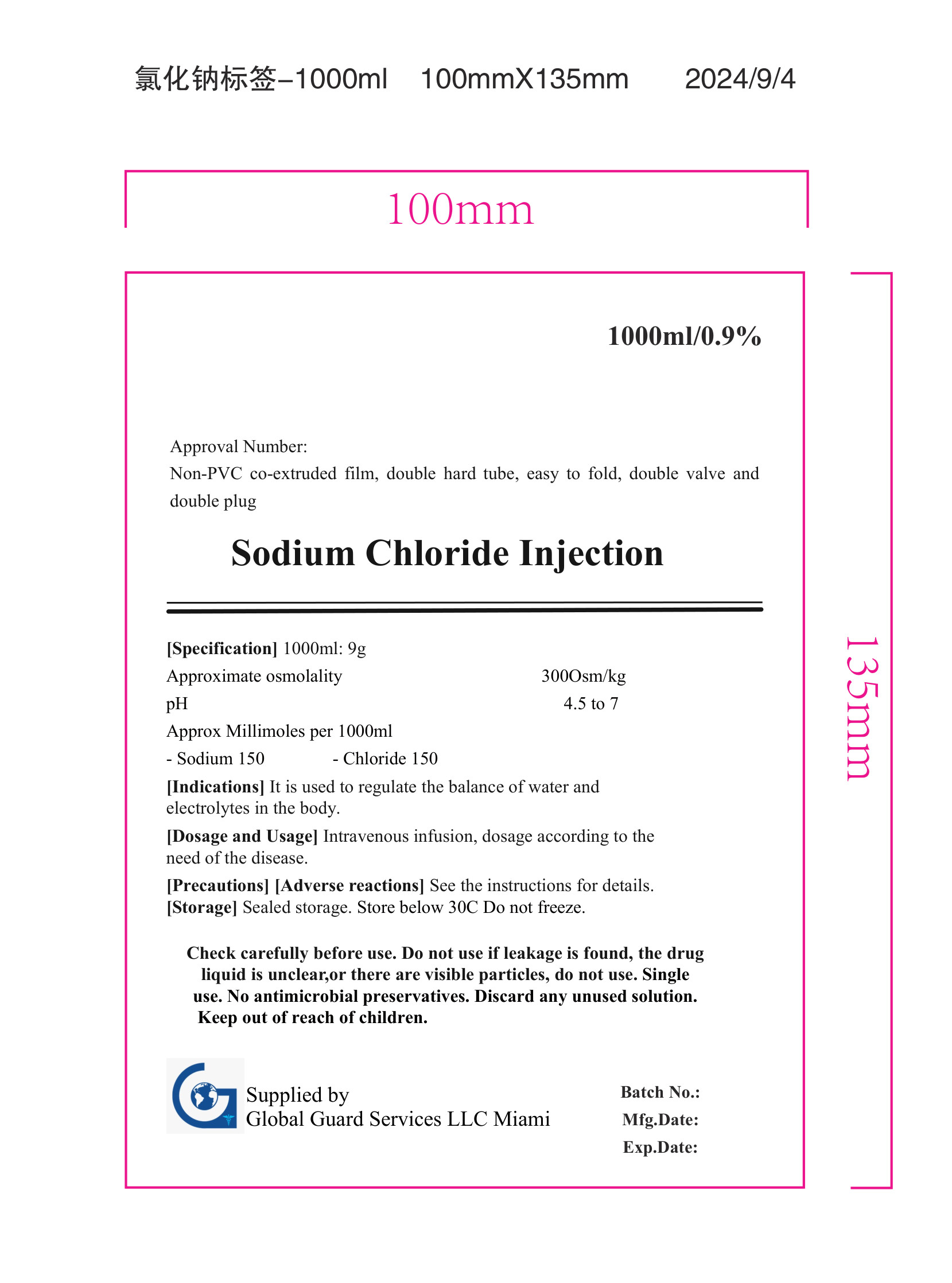 Approval Number:
Approval Number:
Non-PVC co-extruded film, double hard tube, easy to fold, double valve and
double plug
Sodium Chloride Injection
[Specification] 1000ml: 9g
Approximate osmolality
pH
Approx Millimoles per 1000ml
300Osm/kg
4.5 to 7
[Indications] It is used to regulate the balance of water and
electrolytes in the body.
[Dosage and Usage] Intravenous infusion, dosage according to the
need of the disease.
[Precautions] [Adverse reactions] See the instructions for details.
[Storage] Sealed storage. Store below 30C Do not freeze.
Check carefully before use. Do not use if leakage is found, the drug
liquid is unclear, or there are visible particles, do not use. Single
use. No antimicrobial preservatives. Discard any unused solution.
Keep out of reach of children.
Supplied by
Global Guard Services LLC Miami Fla
| SODIUM CHLORIDE
sodium chloride injection, solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Global Guard Services LLC (128182204) |
| Registrant - Shijiazhuang No 4 Pharmaceutical Co Ltd (654175561) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Global Guard Services LLC | 128182204 | label(84845-806) | |
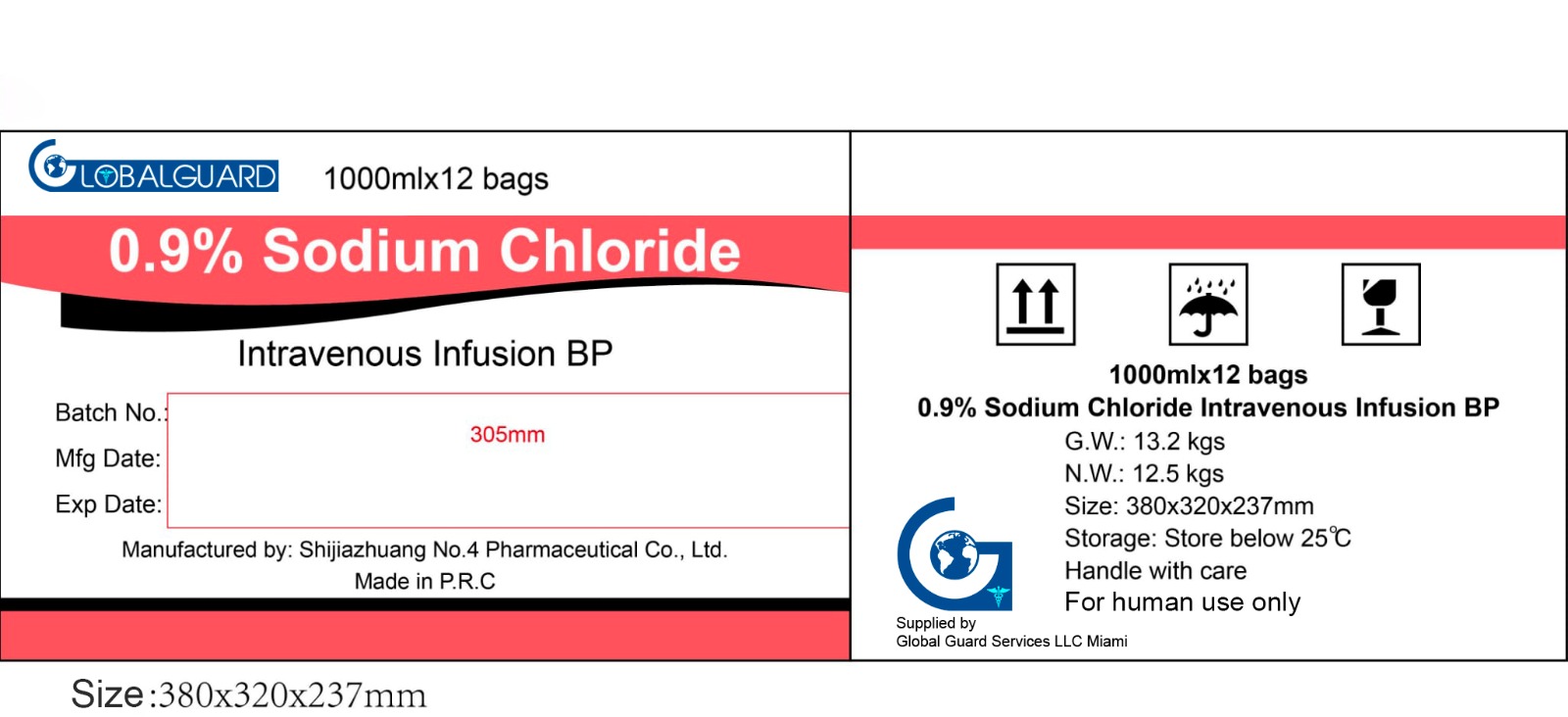 Sodium Chloride Carton for Storage and Delivery
Sodium Chloride Carton for Storage and Delivery