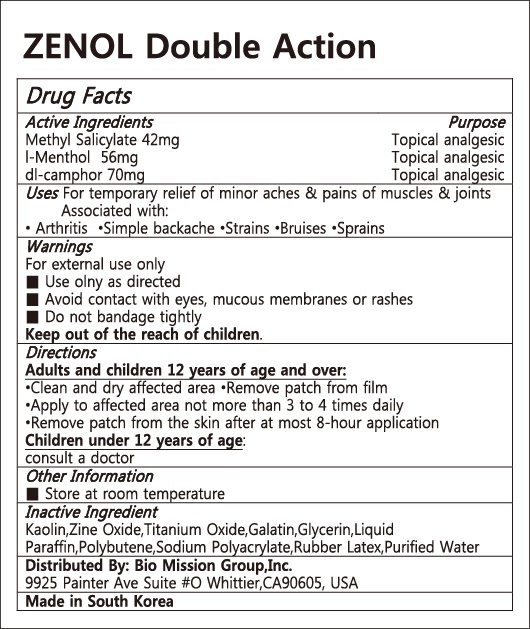
Zenol Double ACTION by Lydia Co., Ltd. Drug Facts
Zenol Double ACTION by
Drug Labeling and Warnings
Zenol Double ACTION by is a Otc medication manufactured, distributed, or labeled by Lydia Co., Ltd.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
ZENOL DOUBLE ACTION- methyl salicylate, l-menthol, dl-camphor patch
Lydia Co., Ltd.
----------
Drug Facts
Kaolin,Zine Oxide,Titanium Oxide,Galatin,Glycerin,Liquid Paraffin,Polybutene,Sodium Polyacrylate,Rubber Latex,Purified Water
| ZENOL DOUBLE ACTION
methyl salicylate, l-menthol, dl-camphor patch |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - Lydia Co., Ltd. (695735569) |
| Registrant - Lydia Co., Ltd. (695735569) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Lydia Co., Ltd. | 695735569 | manufacture(72988-0042) , label(72988-0042) | |
Revised: 12/2024
Document Id: 2973314f-67f6-9466-e063-6394a90a2d58
Set id: 25d26e62-a184-cbed-e063-6294a90afc4f
Version: 2
Effective Time: 20241217
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.