IBRITE- sodium fluoride, potassium nitrate paste, dentifrice
iBrite by
Drug Labeling and Warnings
iBrite by is a Otc medication manufactured, distributed, or labeled by Pac-Dent International Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
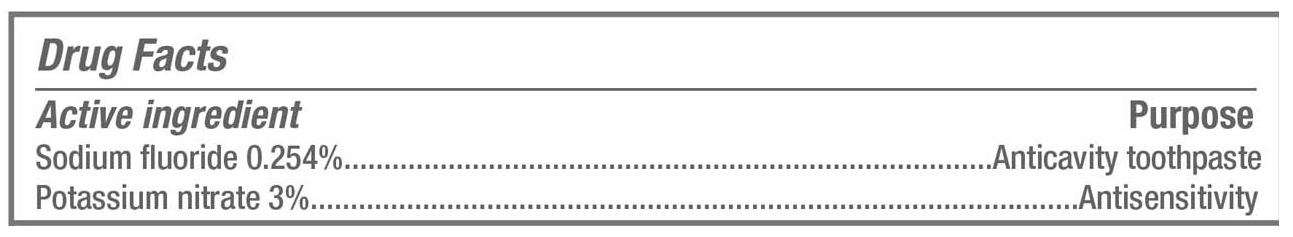
- ACTIVE INGREDIENT
- INACTIVE INGREDIENT
- WARNINGS
- DOSAGE & ADMINISTRATION
- INDICATIONS & USAGE
- PURPOSE
- KEEP OUT OF REACH OF CHILDREN
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
IBRITE
sodium fluoride, potassium nitrate paste, dentifriceProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 68983-006 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM FLUORIDE (UNII: 8ZYQ1474W7) (FLUORIDE ION - UNII:Q80VPU408O) FLUORIDE ION 2.54 mg in 1 g POTASSIUM NITRATE (UNII: RU45X2JN0Z) (NITRATE ION - UNII:T93E9Y2844) POTASSIUM NITRATE 30 mg in 1 g Inactive Ingredients Ingredient Name Strength SACCHARIN SODIUM (UNII: SB8ZUX40TY) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C GREEN NO. 3 (UNII: 3P3ONR6O1S) GLYCERIN (UNII: PDC6A3C0OX) HYDRATED SILICA (UNII: Y6O7T4G8P9) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) WATER (UNII: 059QF0KO0R) SODIUM LAURYL SULFATE (UNII: 368GB5141J) SODIUM PYROPHOSPHATE (UNII: O352864B8Z) SODIUM CITRATE (UNII: 1Q73Q2JULR) SODIUM PHOSPHATE, TRIBASIC (UNII: A752Q30A6X) CARBOXYMETHYLCELLULOSE SODIUM (UNII: K679OBS311) Product Characteristics Color green Score Shape Size Flavor PEPPERMINT Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68983-006-02 1 in 1 CARTON 02/01/2016 1 NDC: 68983-006-01 140 g in 1 TUBE; Type 0: Not a Combination Product 2 NDC: 68983-006-04 1 in 1 CARTON 02/01/2016 2 NDC: 68983-006-03 25 g in 1 TUBE; Type 0: Not a Combination Product 
Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part356 02/01/2016 Labeler - Pac-Dent International Inc. (073253994) Registrant - Pac-Dent International Inc. (073253994) Establishment Name Address ID/FEI Business Operations Pac-Dent International Inc. 073253994 manufacture(68983-006)
Trademark Results [iBrite]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 IBRITE 88450760 not registered Live/Pending |
Pac-Dent, Inc. 2019-05-29 |
 IBRITE 78590877 not registered Dead/Abandoned |
Optec Displays, Inc. 2005-03-18 |
 IBRITE 77548951 not registered Dead/Abandoned |
Alan Austin Creamer 2008-08-17 |
 IBRITE 77133767 3610843 Live/Registered |
PAC-DENT, INC. 2007-03-17 |
 IBRITE 76155840 2799619 Dead/Cancelled |
ibrite, Inc. 2000-10-30 |
 IBRITE 75794142 2464513 Dead/Cancelled |
iBrite, Inc. 1999-09-09 |
 IBRITE 75794141 2454060 Dead/Cancelled |
ibrite, Inc. 1999-09-09 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.