G.M. COLLIN MINERAL SUN VEIL- zinc oxide cream
G.M. Collin Mineral Sun Veil by
Drug Labeling and Warnings
G.M. Collin Mineral Sun Veil by is a Otc medication manufactured, distributed, or labeled by Laboratoires Dermo Cosmetik Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- PhytoWhite™ Dark Spot Serum
- SPL UNCLASSIFIED SECTION
- SPL UNCLASSIFIED SECTION
- SPL UNCLASSIFIED SECTION
- SPL UNCLASSIFIED SECTION
- SPL UNCLASSIFIED SECTION
- SPL UNCLASSIFIED SECTION
- SPL UNCLASSIFIED SECTION
- SPL UNCLASSIFIED SECTION
- SPL UNCLASSIFIED SECTION
-
INGREDIENTS
WATER/EAU, SD ALCOHOL 3-A, BUTYLENE GLYCOL, GLYCOLIC ACID, ARGININE, POTASSIUM AZELOYL DIGLYCINATE, GLYCERIN, BRASSICA NAPUS EXTRACT, NIACINAMIDE, 1-METHYLHYDANTOINE-2-IMIDE, POLYSORBATE 20, ETHOXYDIGLYCOL, PHYLLANTHUS EMBLICA FRUIT EXTRACT, ERGOTHIONEINE, TETRAHYDRODIFERULOYLMETHANE, LICORICE EXTRACT, POTASSIUM ASCORBYL TOCOPHERYL PHOSPHATE (VITAMIN C, VITAMIN E), ALLANTOIN, HYDROXYPROPYLCELLULOSE, TETRAHYDROPIPERINE, PEG-40 HYDROGENATED CASTOR OIL, DISODIUM EDTA, SODIUM METABISULFITE, FRAGRANCE/PARFUM
- SPL UNCLASSIFIED SECTION
- SPL UNCLASSIFIED SECTION
-
INGREDIENTS
WATER/EAU, OLIGOPEPTIDE-34, ARACHIDYL ALCOHOL, BEHENYL ALCOHOL, ARACHIDYL GLUCOSIDE, NIACINAMIDE, BRASSICA NAPUS EXTRACT, PENTYLENE GLYCOL, GLYCERIN, DICAPRYLYL CARBONATE, SODIUM PCA, UREA, TREHALOSE, POLYQUATERNIUM-51, SODIUM HYALURONATE, LEPIDIUM SATIVUM SPROUT EXTRACT, PHENOXYETHANOL, LECITHIN, SOY ISOFLAVONES, POLYSORBATE 80 , ALCOHOL, SQUALANE (VEGETAL), PEG-8, CETEARYL ISONONANOATE, ASCORBYL GLUCOSIDE, PICHIA/RESVERATROL FERMENT EXTRACT, TOCOPHERYL ACETATE (VITAMIN E), ISOSTEARYL AVOCADATE, BUTYROSPERMUM PARKII (SHEA BUTTER), BIOSACCHARIDE GUM-1, DIPALMITOYL HYDROXYPROLINE, DIMETHICONE, SODIUM ACRYLATE/SODIUM ACRYLOYLDIMETHYL TAURATE COPOLYMER, ISOHEXADECANE,CYCLOPENTASILOXAN E, CYCLOTETRASILOXANE, DIMETHICONOL, PEG-100 STEARATE, GLYCERYL STEARATE, ERGOTHIONEINE, ASCORBYL TETRA 2-HEXYLDECANOATE, XYLITYLGLUCOSIDE, ANHYDROXYLITOL, XYLITOL, CAPRYLIC/CAPRIC TRIGLYCERIDE, SOYBEAN (GLYCINE SOYA), PANTHENOL, C12-15 ALKYL BENZOATE, TRIBEHENIN, CERAMIDE 2, PEG-10 RAPESEED STEROL, PALMITOYL OLIGOPEPTIDE, FRAGRANCE/PARFUM, CHLORPHENESIN, ETHYLHEXYLGLYCERIN, SODIUM METABISULFITE, DISODIUM EDTA, SODIUM HYDROXIDE, SODIUM CITRATE, TETRADIBUTYL PENTAERITHRITYL HYDROXYHYDROCINNAMATE, XANTHAN GUM, CITRIC ACID
-
SPL UNCLASSIFIED SECTION
15 SPF MINERAL SUN VEIL CREAM
NDC: 64127-014-02 - SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENTS
-
INGREDIENTS
CYCLOPENTASILOXANE, CYCLOHEXASILOXANE, PEG-10 DIMETHICONE, POLYSILICONE-11, WATER/EAU, DIMETHICONE/DIVINYLDIMETHICONE/SILSESQUIOXANE CROSSPOLYMER, COPERNICIA CERIFERA (CARNAUBA) WAX, PPG-15 STEARYL ETHER, CETYL PEG/PPG-10/1 DIMETHICONE, GLYCERIN, BUTYLENE GLYCOL, CITRUS MEDICA LIMONIUM (LEMON) EXTRACT, FUMARIA OFFICINALIS EXTRACT, FUMARIC ACID, SODIUM BENZOTRIAZOLYL BUTYLPHENOL SULFONATE, BUTETH-3, TRIBUTYL CITRATE, TRIS (TETRAMETHYLHYDROXYPIPERIDINOL) CITRATE, ETHYLHEXYLGLYCERIN, CHLORPHENESIN, CASSIA ALATA (CANDLE TREE) LEAF EXTRACT, TOCOPHEROL (VITAMIN E)
- SPL UNCLASSIFIED SECTION
-
PRINCIPAL DISPLAY PANEL - Kit Carton
SYSTÈME ÉCLAIRCISSANT
PHYTOWHITE™
BRIGHTENING SYSTEMTous types de peau
All Skin TypesNDC: 64127-084-01
SÉRUM
ANTI-TACHES
PHYTOWHITE™
DARK SPOT
SERUM
20 ml – 0.68 fl. oz.CRÈME
PHYTOWHITE™
CREAM
50 ml – 1.7 fl. oz.VOILE SOLAIRE
MINERAL
SUN VEIL
FPS 15 SPF
50 ml – Net wt. 1.7 oz.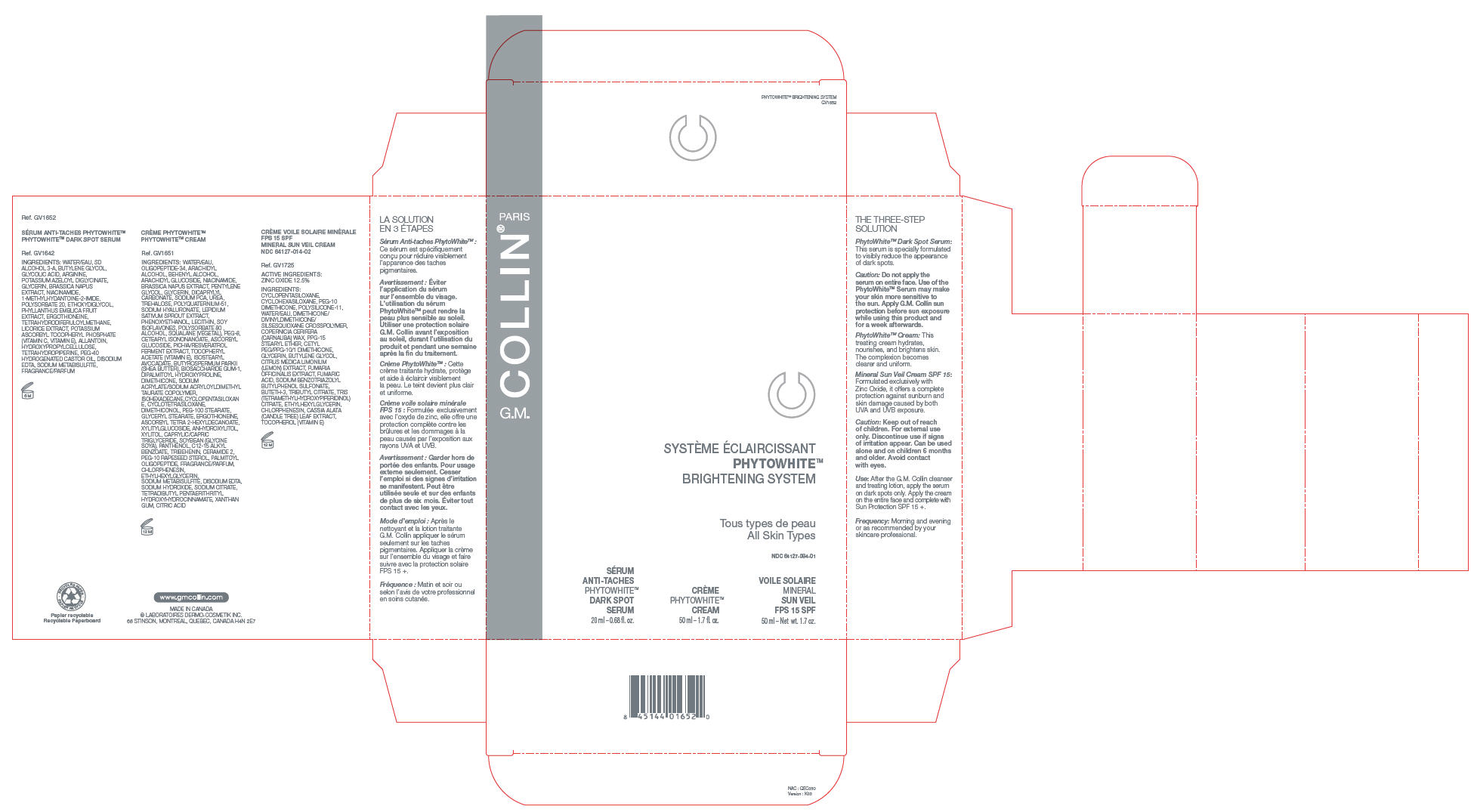
-
INGREDIENTS AND APPEARANCE
G.M. COLLIN MINERAL SUN VEIL
zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 64127-084 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength zinc oxide (UNII: SOI2LOH54Z) (zinc oxide - UNII:SOI2LOH54Z) zinc oxide 0.125 mL in 1 mL Inactive Ingredients Ingredient Name Strength CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) CYCLOMETHICONE 6 (UNII: XHK3U310BA) WATER (UNII: 059QF0KO0R) CARNAUBA WAX (UNII: R12CBM0EIZ) GLYCERIN (UNII: PDC6A3C0OX) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) LEMON (UNII: 24RS0A988O) FUMARIC ACID (UNII: 88XHZ13131) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) CHLORPHENESIN (UNII: I670DAL4SZ) ALPHA-TOCOPHEROL (UNII: H4N855PNZ1) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 64127-084-01 1 in 1 KIT 1 50 mL in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 05/17/2010 Labeler - Laboratoires Dermo Cosmetik Inc. (249335480) Establishment Name Address ID/FEI Business Operations Laboratoires Dermo Cosmetik Inc. 249335480 MANUFACTURE
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.