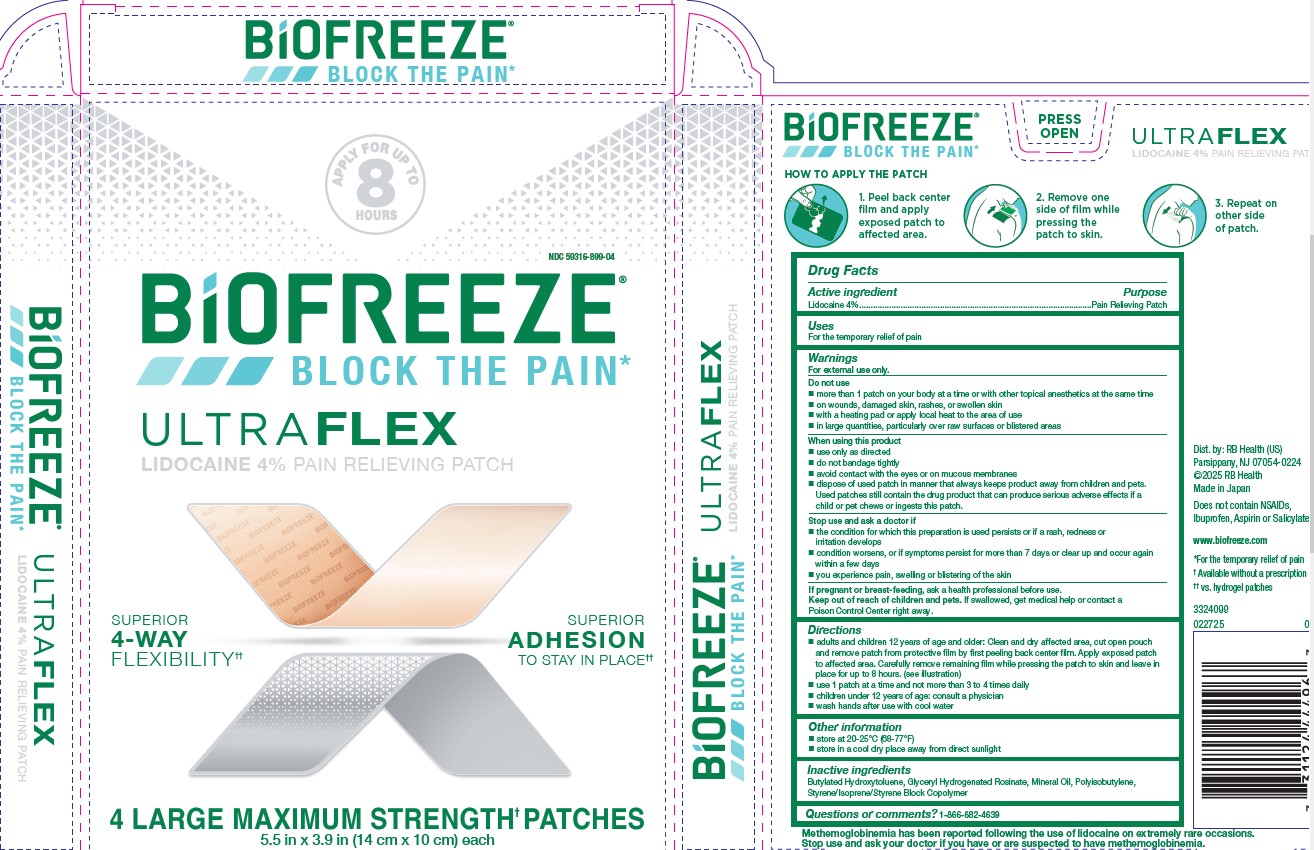
BIOFREEZE ULTRAFLEX- lidocaine patch
Biofreeze Ultraflex by
Drug Labeling and Warnings
Biofreeze Ultraflex by is a Otc medication manufactured, distributed, or labeled by RB Health (US) LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- ACTIVE INGREDIENT
- Uses
-
WARNINGS
Warnings
For external use only.
Do not use
more than 1 patch on your body at a time or with other topical anesthetics at the same time
on wounds, damaged skin, rashes, or swollen skin
with a heating pad or apply local heat to the area of use
in large quantities, particularly over raw surfaces or blistered areas
When using this product
use only as directed
do not bandage tightly
avoid contact with the eyes or on mucous membranes
dispose of used patch in manner that always keeps product away from children and pets.
Used patches still contain the drug product that can produce serious adverse effects if a
child or pet chews or ingests this patch.
Stop use and ask a doctor if
the condition for which this preparation is used persists or if a rash, redness or
irritation develops
condition worsens, or if symptoms persist for more than 7 days or clear up and occur again
within few days
you experience pain, swelling or blistering of the skin
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children and pets. If swallowed, get medical help or contact a
Poison Control Center right away. - INDICATIONS & USAGE
- KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
Directions
adults and children 12 years of age and older: Clean and dry affected area, cut open pouch
and remove patch from protective film by first peeling back center film. Apply exposed patch
to affected area. Carefully remove remaining film while pressing the patch to skin and leave in
place for up to 8 hours. (see images on carton)
use 1 patch at a time and not more than 3 to 4 times daily
children under 12 years of age: consult a physician
wash hands after use with cool water
- OTHER SAFETY INFORMATION
- INACTIVE INGREDIENT
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
BIOFREEZE ULTRAFLEX
lidocaine patchProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 59316-899 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE (UNII: 98PI200987) (LIDOCAINE - UNII:98PI200987) LIDOCAINE 0.04 g in 1 g Inactive Ingredients Ingredient Name Strength GLYCEROL ESTER OF HYDROGENATED ROSIN (UNII: XH62RP786N) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) POLYISOBUTYLENE (55000 MW) (UNII: TQ77WR8A02) STYRENE/ISOPRENE/STYRENE BLOCK COPOLYMER (UNII: K7S96QM8DV) MINERAL OIL (UNII: T5L8T28FGP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 59316-899-04 4 in 1 CARTON 01/15/2025 1 2 g in 1 PATCH; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) 2 NDC: 59316-899-07 1 in 1 POUCH 01/15/2025 2 2 g in 1 PATCH; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 01/15/2025 Labeler - RB Health (US) LLC (081049410)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.