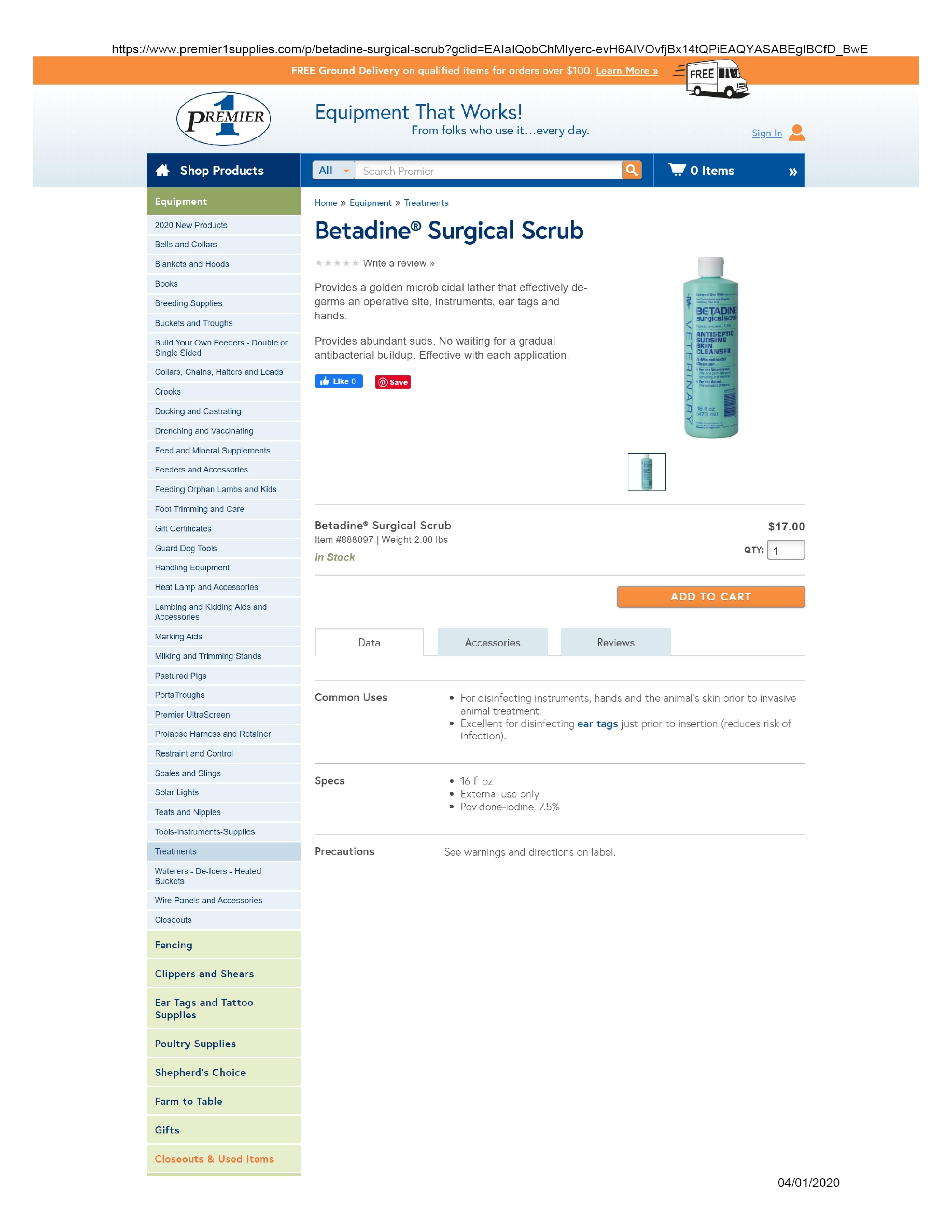
7.5% Betadine Surgical Scrub 4oz and 1 gallon
Betadine by
Drug Labeling and Warnings
Betadine by is a Otc medication manufactured, distributed, or labeled by Xttrium Laboratories, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
BETADINE- povidone-iodine liquid
Xttrium Laboratories, Inc.
----------
7.5% Betadine Surgical Scrub 4oz and 1 gallon
Uses
■ for preparation of the skin prior to surgery
■ helps to reduce bacteria that potentially can cause skin infection
■ for handwashing to reduce bacteria on the skin
■ significantly reduces the number of microorganisms on the hands forearms prior to surgery or patient care
Warnings
For external use only
Do not use
■ in the eyes
When using this product ■ prolonged exposure to wet solution may cause irritation or, rarely, severe skin reactions
■ in pre-operative prepping, avoid "pooling" beneath the patient
Stop use and ask a doctor ■ if irritation and redness develop
■ in rare instances of local irritation or sensitivity
Directions
A. Surgical hand scrub:
- wet hands with water
- spread about 5 cc (1 teaspoonful) of Scrub over both handsand forearms
- without adding more water, scrub thoroughly for 2 1/2 to3 minutes
- use a sponge if desired. Clean thoroughly under fingernails.
- add a little water and develop copious suds. Rinse thoroughlyunder running water.
- repeat the entire procedure using another 5 cc of Scrub
B. Antiseptic hand wash:
- wet hands with water and pour about 5 cc of Scrub on hands
- rub hands vigorously together for at least 15 seconds, coveringall surfaces
- rinse and dry with a disposable towel
C. Patient pre-operative skin preparation:
■ single use only
■ wet skin with water
■ apply Scrub (1 cc is sufficient to cover an area of 20-30 square inches),
develop lather and scrub thoroughly for about 5 minutes
■ rinse off using sterile gauze saturated with water
■ the area may then be painted with BETADINE Solution and allowed to dry
| BETADINE
povidone-iodine liquid |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Xttrium Laboratories, Inc. (007470579) |
Trademark Results [Betadine]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 BETADINE 90851747 not registered Live/Pending |
Avrio Health L.P. 2021-07-27 |
 BETADINE 75438619 2289922 Live/Registered |
AVRIO HEALTH L.P. 1998-02-23 |
 BETADINE 75429382 not registered Dead/Abandoned |
PURDUE FREDERICK COMPANY, THE 1998-02-05 |
 BETADINE 75429381 2649701 Dead/Cancelled |
PURDUE FREDERICK COMPANY, THE 1998-02-05 |
 BETADINE 75429376 2225977 Live/Registered |
AVRIO HEALTH L.P. 1998-02-05 |
 BETADINE 75000809 1984349 Dead/Cancelled |
PURDUE FREDERICK COMPANY, THE 1995-10-02 |
 BETADINE 74711684 1984228 Dead/Cancelled |
PURDUE FREDERICK COMPANY, THE 1995-08-07 |
 BETADINE 74596982 1982032 Dead/Cancelled |
Purdue Frederick Company, The 1994-11-09 |
 BETADINE 73839535 1601849 Live/Registered |
PURDUE FREDERICK COMPANY 1989-11-15 |
 BETADINE 73779372 1559504 Live/Registered |
PURDUE FREDERICK COMPANY, THE 1989-02-06 |
 BETADINE 73107412 1069261 Dead/Expired |
PURDUE FREDERICK COMPANY, THE 1976-11-22 |
 BETADINE 73072465 1059771 Dead/Expired |
PURDUE FREDERICK COMPANY, THE 1975-12-22 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.

