Betadine by Xttrium Laboratories, Inc. 0.5% Betadine Gargle 8oz
Betadine by
Drug Labeling and Warnings
Betadine by is a Otc medication manufactured, distributed, or labeled by Xttrium Laboratories, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
BETADINE- povidone iodine liquid
Xttrium Laboratories, Inc.
----------
0.5% Betadine Gargle 8oz
Warning
Sore Throat Warning: Severe or persistent sore throat or sore throat accompanied by high
fever, headache, nausea, and vomiting may be serious. Consult a doctor promptly. Do not use more than
2 days or administer to children under 3 years of age unless directed by a doctor.
Do not use this product
■ for more than 7 days unless directed by a dentist or doctor
■ if you are allergic to povidone-iodine or any othe ringredients in this product
■ if you have any thyroid condtions
When using this product
■ do not get into eyes. If contact occurs, rinse eyes thoroughly with water. If irritation persists, consult a doctor
■ do not swallow
■ do not use with hydrogen peroxide
Stop use and ask a doctor
■ symptoms do not improve in 7 days
■ irritation, pain, or redness persists or worsens
■ swelling, rash, or fever develops
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children. In case more than used for gargling is accidentally swallowed, get medical
help or contact a Poison Control Center right away.
Directions
DO NOT SWAllOW
Adults and children12 years and older:
■ Gargle with 10 ml full strength for 30 seconds, then spit out
■ Use only with dosing cup provided
■ Use up to 4 times daily
Children under 12: Do not use.
Other information
■ store at 25°F (77°F); excursions permitted between 15°-30°C (59°-86°F)
■ may stain clothing or dental work
■ avoid contact with jewelry, especially silver
| BETADINE
povidone iodine liquid |
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
| Labeler - Xttrium Laboratories, Inc. (007470579) |
Trademark Results [Betadine]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 BETADINE 90851747 not registered Live/Pending |
Avrio Health L.P. 2021-07-27 |
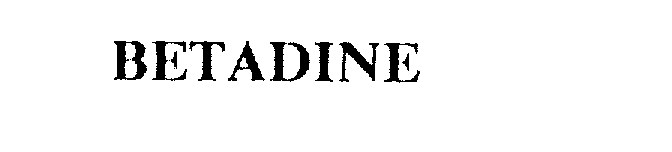 BETADINE 75438619 2289922 Live/Registered |
AVRIO HEALTH L.P. 1998-02-23 |
 BETADINE 75429382 not registered Dead/Abandoned |
PURDUE FREDERICK COMPANY, THE 1998-02-05 |
 BETADINE 75429381 2649701 Dead/Cancelled |
PURDUE FREDERICK COMPANY, THE 1998-02-05 |
 BETADINE 75429376 2225977 Live/Registered |
AVRIO HEALTH L.P. 1998-02-05 |
 BETADINE 75000809 1984349 Dead/Cancelled |
PURDUE FREDERICK COMPANY, THE 1995-10-02 |
 BETADINE 74711684 1984228 Dead/Cancelled |
PURDUE FREDERICK COMPANY, THE 1995-08-07 |
 BETADINE 74596982 1982032 Dead/Cancelled |
Purdue Frederick Company, The 1994-11-09 |
 BETADINE 73839535 1601849 Live/Registered |
PURDUE FREDERICK COMPANY 1989-11-15 |
 BETADINE 73779372 1559504 Live/Registered |
PURDUE FREDERICK COMPANY, THE 1989-02-06 |
 BETADINE 73107412 1069261 Dead/Expired |
PURDUE FREDERICK COMPANY, THE 1976-11-22 |
 BETADINE 73072465 1059771 Dead/Expired |
PURDUE FREDERICK COMPANY, THE 1975-12-22 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.
