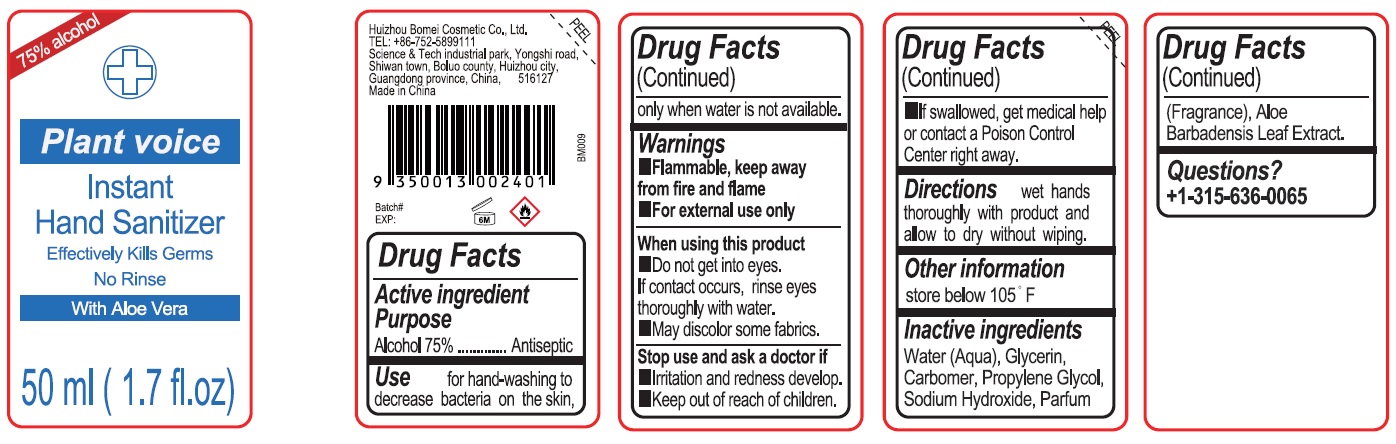
BM009 Plant Voice Hand sanitizer
BM009 Plant Voice Hand sanitizer by
Drug Labeling and Warnings
BM009 Plant Voice Hand sanitizer by is a Otc medication manufactured, distributed, or labeled by Huizhou Bomei Cosmetic Co., Ltd.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
BM009 PLANT VOICE HAND SANITIZER- alcohol gel
Huizhou Bomei Cosmetic Co., Ltd.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
BM009 Plant Voice Hand sanitizer
Warnings
- Flammable, keep away from fire and flame
- For external use only
| BM009 PLANT VOICE HAND SANITIZER
alcohol gel |
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
| Labeler - Huizhou Bomei Cosmetic Co., Ltd. (546012568) |
Revised: 12/2021
Document Id: d23fa6d5-e40a-0c7c-e053-2a95a90ac2ac
Set id: 2671b9b5-152f-4309-b725-5a6636af143c
Version: 3
Effective Time: 20211203