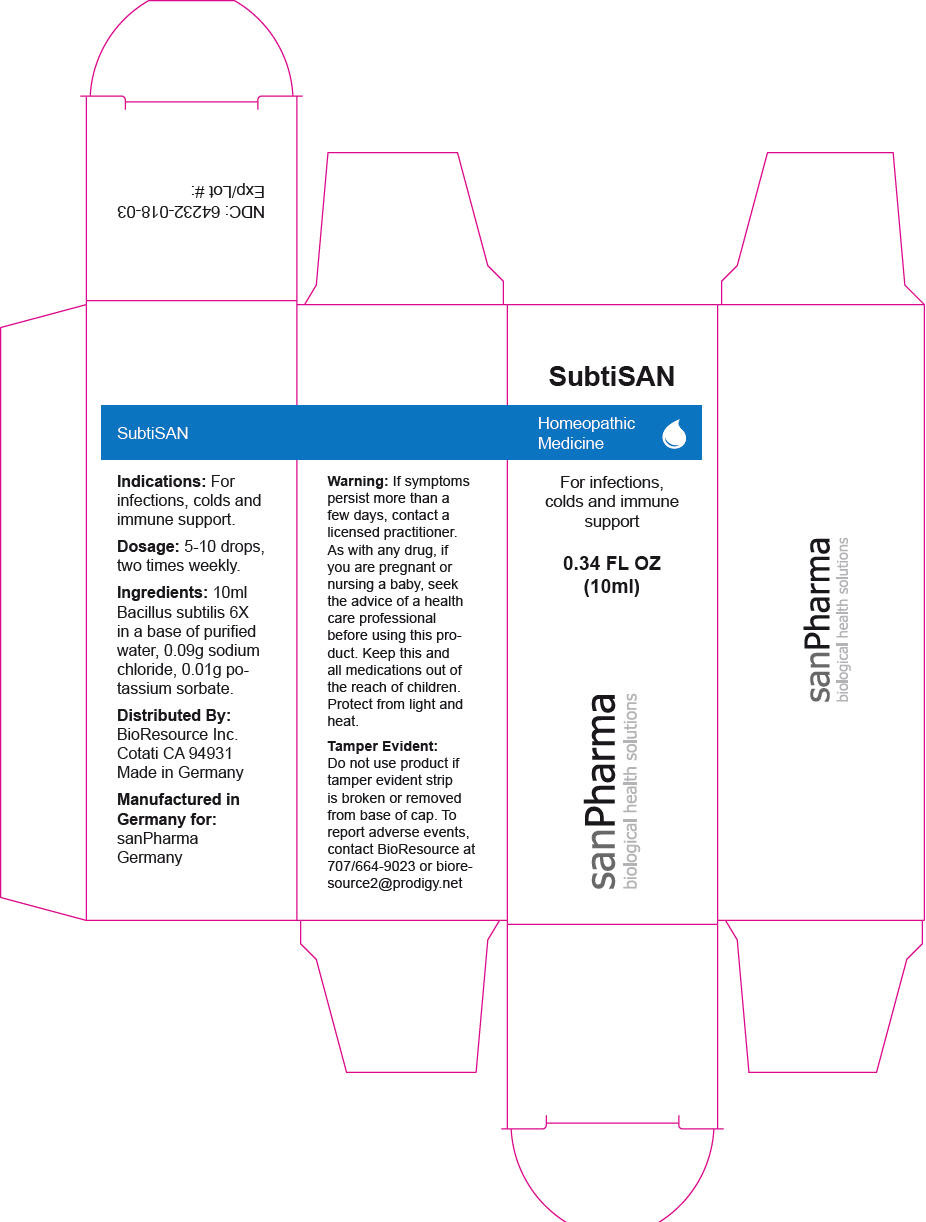
SubtiSAN by sanPharma GmbH SubtiSAN
SubtiSAN by
Drug Labeling and Warnings
SubtiSAN by is a Homeopathic medication manufactured, distributed, or labeled by sanPharma GmbH. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
SUBTISAN- bacillus subtilis liquid
sanPharma GmbH
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
SubtiSAN
Warning
If symptoms persist more than a few days, contact a licensed practitioner. As with any drug, if you are pregnant or nursing a baby, seek the advice of a health care professional before using this product.
| SUBTISAN
bacillus subtilis liquid |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - sanPharma GmbH (341409153) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| sanPharma GmbH | 341409153 | manufacture(64232-018) , label(64232-018) | |
Revised: 12/2019
Document Id: 995792f6-d680-db60-e053-2995a90ad154
Set id: 267afcc4-cfe1-4b8b-b18f-fa035e396db6
Version: 4
Effective Time: 20191210
sanPharma GmbH