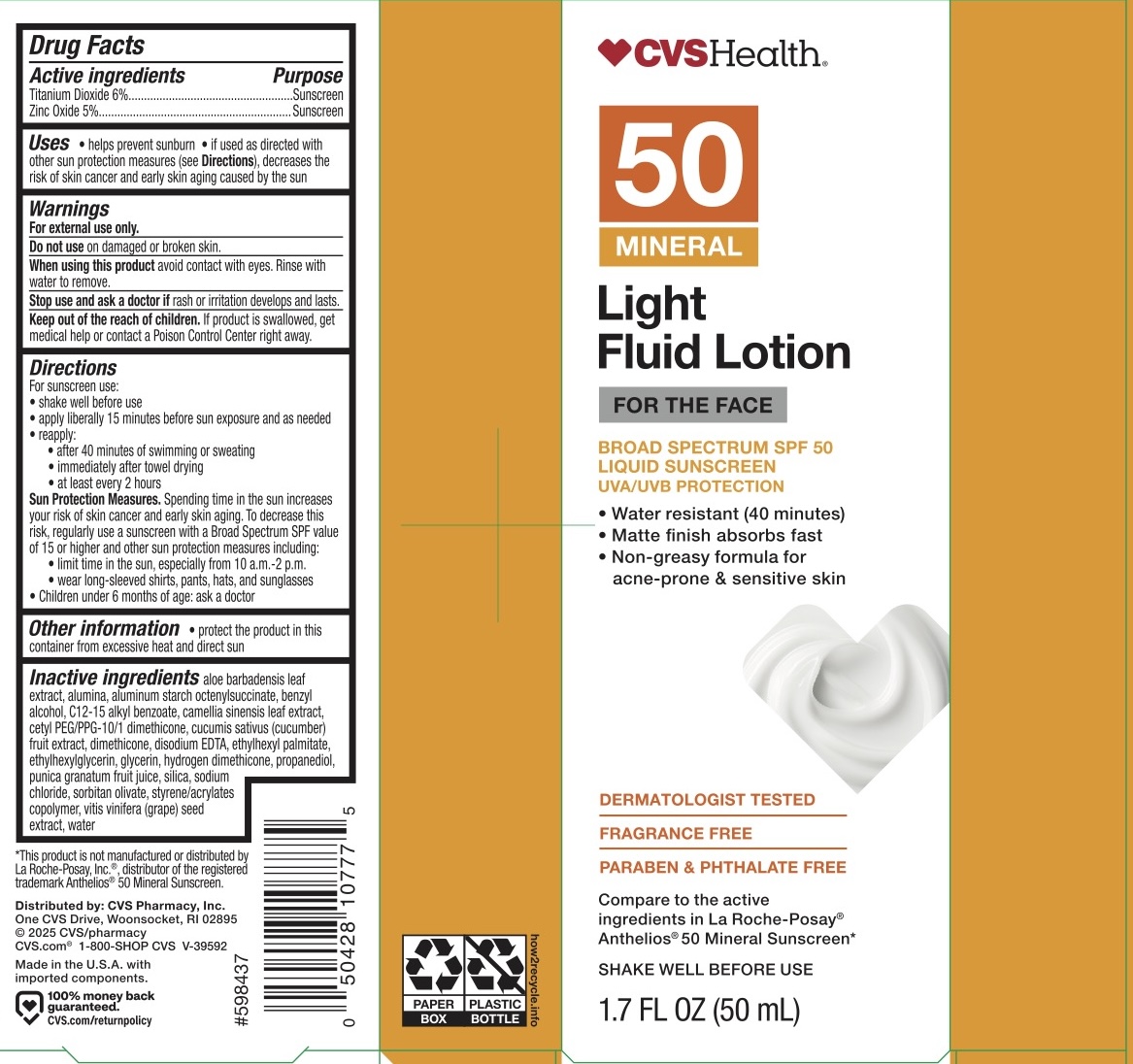
CVS SPF 50 FACE SUNSCREEN- titanium dioxide, zinc oxide lotion
CVS SPF 50 Face Sunscreen by
Drug Labeling and Warnings
CVS SPF 50 Face Sunscreen by is a Otc medication manufactured, distributed, or labeled by CVS, Derma Care Research Labs, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
For sunscreen use: shake well before use, apply liberally 15 minutes before sun exposure and as needed, reapply: after 40 minutes of swimming or sweating, immediately after towel drying, and at least every 2 hours.
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including: limit time in the sun, especially from 10 am - 2 pm, wear long-sleeved shirts, pants, hats, and sunglasses.
Children under 6 months of age: ask a doctor.
-
INACTIVE INGREDIENT
Aloe barbadensis leaf extract, alumina, aluminum starch octenylsuccinate, benzyl alcohol, C12-15 alkyl benzoate, camellia sinensis leaf extract, cetyl PEG/PPG-10/1 dimethicone, cucumis sativus (cucumber) fruit extract, dimethicone, disodium EDTA, ethyhexyl palmitate, ethyhexylglycerin, glycerin, hydrogen dimethicone, propanediol, punica granatum fruit juice, silica, sodium chloride, sorbitan olivate, styrene/acrylates copolymer, vitis vinifera (grape) seed extract, water.
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CVS SPF 50 FACE SUNSCREEN
titanium dioxide, zinc oxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 51316-217 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 5 g in 100 mL TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 6 g in 100 mL Inactive Ingredients Ingredient Name Strength ALUMINUM STARCH OCTENYLSUCCINATE (UNII: I9PJ0O6294) GLYCERIN (UNII: PDC6A3C0OX) HYDROGEN DIMETHICONE (40 CST) (UNII: T8RBB3RCP9) PROPANEDIOL (UNII: 5965N8W85T) SODIUM CHLORIDE (UNII: 451W47IQ8X) WATER (UNII: 059QF0KO0R) EDETATE DISODIUM (UNII: 7FLD91C86K) ETHYLHEXYL PALMITATE (UNII: 2865993309) BENZYL ALCOHOL (UNII: LKG8494WBH) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) STYRENE/ACRYLAMIDE COPOLYMER (MW 500000) (UNII: 5Z4DPO246A) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) ALUMINA (UNII: LMI26O6933) CAMELLIA SINENSIS LEAF (UNII: W2ZU1RY8B0) CUCUMBER (UNII: YY7C30VXJT) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 2) (UNII: V2W71V8T0X) PUNICA GRANATUM FRUIT JUICE (UNII: 99S671U9KB) SORBITAN OLIVATE (UNII: MDL271E3GR) ALOE VERA LEAF (UNII: ZY81Z83H0X) VITIS VINIFERA (GRAPE) SEED (UNII: C34U15ICXA) DIMETHICONE 200 (UNII: RGS4T2AS00) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 51316-217-01 1 in 1 BOX 01/28/2025 1 50 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 01/28/2025 Labeler - CVS (062312574) Registrant - Derma Care Research Labs, LLC (116817470) Establishment Name Address ID/FEI Business Operations Derma Care Research Labs, LLC 116817470 manufacture(51316-217)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.