YouCopia Pain Relief Cream by Good Manager Holdings Inc. 82372-014 Complete
YouCopia Pain Relief Cream by
Drug Labeling and Warnings
YouCopia Pain Relief Cream by is a Otc medication manufactured, distributed, or labeled by Good Manager Holdings Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
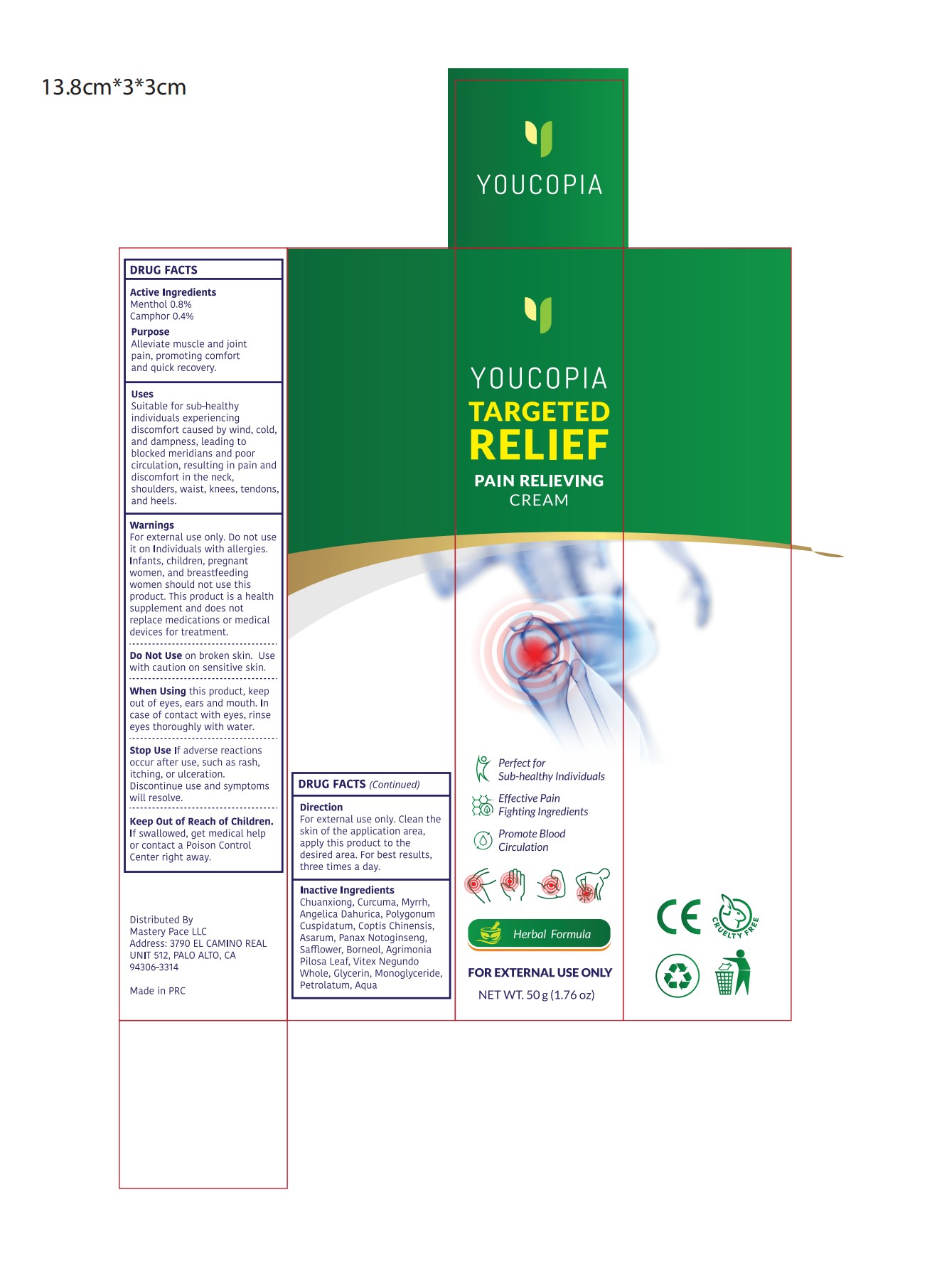
YOUCOPIA PAIN RELIEF CREAM- pain relief cream cream
Good Manager Holdings Inc.
----------
82372-014 Complete
Use
Suitable for sub-healthyindividuals experiencingdiscomfort caused by wind, coldand dampness, leading toblocked meridians and poorcirculation, resulting in pain anddiscomfort in the neck,shoulders, waist, knees, tendons.and heels.
Warnings
For external use only.
Do not useit on Individuals with allergies.
Infants, children, pregnantwomen,and breastfeedingwomen should not use thisproduct.
This product is a healthsupplement and does notreplace medications or medicaldevices for treatment.
When Using
when Using this product, keepout of eyes,ears and mouth. Incase of contact with eyes, rinseeyes thoroughly with water.
Stop Use
Stop Use lf adverse reactionsoccur after use,such as rash,itching, or ulceration.
Discontinue use and symptomswill resolve.
Keep Oot Of Reach Of Children
If swallowed, get medical helpor contact a Poison ControlCenter right away.
Directions
For external use only.
Clean theskin of the application area, apply this product to thedesired area, For best results,three times a day.
| YOUCOPIA PAIN RELIEF CREAM
pain relief cream cream |
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
| Labeler - Good Manager Holdings Inc. (118382673) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Good Manager Holdings Inc. | 118382673 | label(82372-014) | |