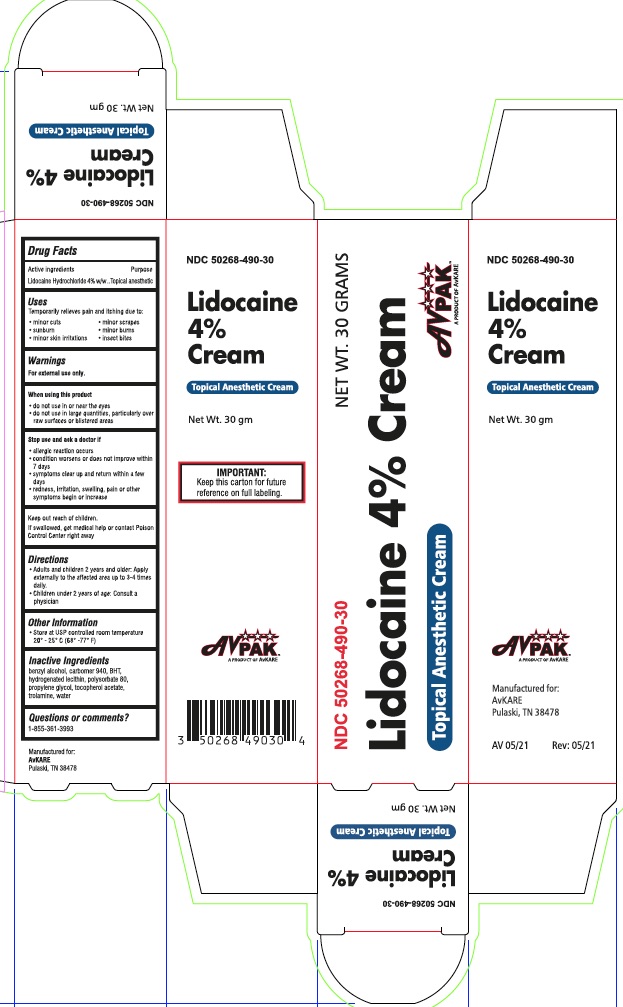
Lidocaine HCl 4% by AvPAK / Denison Pharmaceuticals Lidocaine HCl 4%
Lidocaine HCl 4% by
Drug Labeling and Warnings
Lidocaine HCl 4% by is a Otc medication manufactured, distributed, or labeled by AvPAK, Denison Pharmaceuticals. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
LIDOCAINE HCL 4%- lidocaine hcl 4% cream
AvPAK
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Lidocaine HCl 4%
Uses
temporarily relieves pain and itching due to:
- minor cuts
- minor scrapes
- sunburn
- minor skin irritations
- minor burns
- insect bites
WARNINGS
For external use only.
When using this product
- do not use in or near the eyes
- do not use in large quantities, particularly over raw surfaces or blistered areas
Directions
- Adults and children 2 years and older: Apply externally to the affected area up to 3-4 times daily.
- Children under 2 years of age: Consult a doctor.
| LIDOCAINE HCL 4%
lidocaine hcl 4% cream |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - AvPAK (832926666) |
| Registrant - Denison Pharmaceuticals (001207208) |
Revised: 1/2023
Document Id: f1feda78-c7d3-45ea-e053-2995a90a7df3
Set id: 270ed9fb-cbfe-4fad-a2ab-2cef154e1a60
Version: 3
Effective Time: 20230111
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.