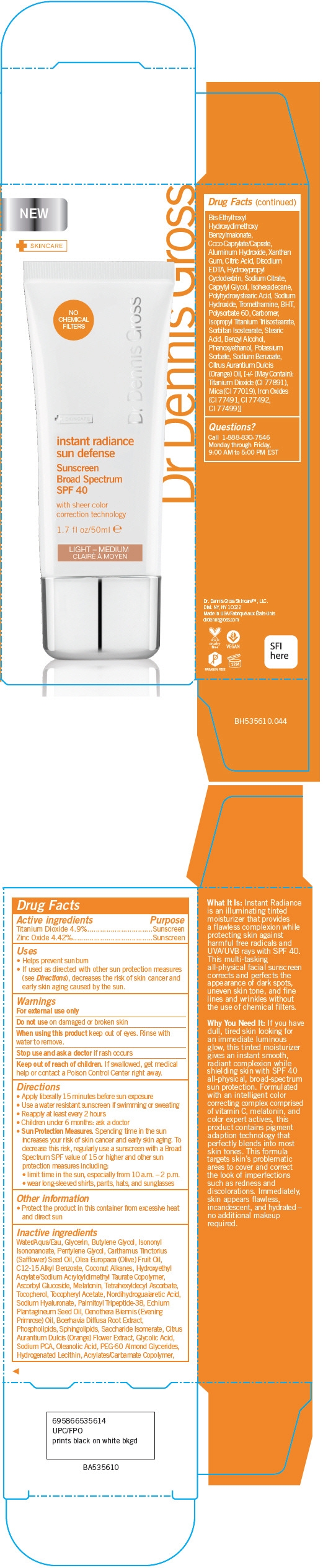
INSTANT RADIANCE SUN DEFENSE SUNSCREEN BROAD SPECTRUM SPF 40 (LIGHT - MEDIUM) WITH SHEER COLOR CORRECTION TECHNOLOGY- titanium dioxide and zinc oxide cream
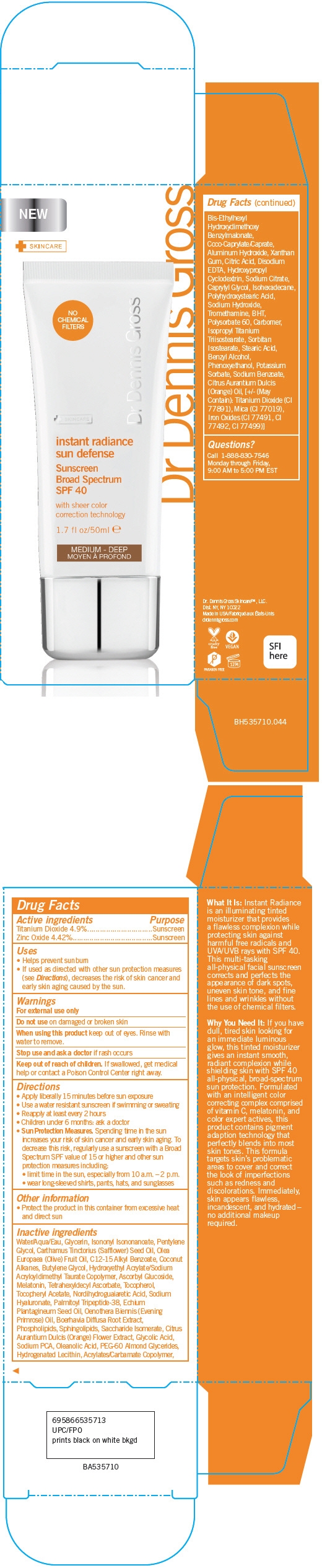
INSTANT RADIANCE SUN DEFENSE SUNSCREEN BROAD SPECTRUM SPF 40 (MEDIUM-DEEP) WITH SHEER COLOR CORRECTION TECHNOLOGY- titanium dioxide and zinc oxide cream
Dr. Dennis Gross Skincare, LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
| Active ingredients | Purpose |
| Titanium Dioxide 4.9% | Sunscreen |
| Zinc Oxide 4.42% | Sunscreen |
Uses
- Helps prevent sunburn
- If used as directed with other sun protection measures (see
Directions), decreases the risk of skin cancer and early skin aging caused by the sun.
Warnings
For external use only
Do not use on damaged or broken skin
When using this product keep out of eyes. Rinse with water to remove.
Stop use and ask a doctor if rash occurs
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- Apply liberally 15 minutes before sun exposure
- Use a water resistant sunscreen if swimming or sweating
- Reapply at least every 2 hours
- Children under 6 months: ask a doctor
-
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m. – 2 p.m.
- wear long-sleeved shirts, pants, hats, and sunglasses
Other information
- Protect the product in this container from excessive heat and direct sun
Inactive ingredients
Water/Aqua/Eau, Glycerin, Butylene Glycol, Isononyl Isononanoate, Pentylene Glycol, Carthamus Tinctorius (Safflower) Seed Oil, Olea Europaea (Olive) Fruit Oil, C12-15 Alkyl Benzoate, Coconut Alkanes, Hydroxyethyl Acrylate/Sodium Acryloyldimethyl Taurate Copolymer, Ascorbyl Glucoside, Melatonin, Tetrahexyldecyl Ascorbate, Tocopherol, Tocopheryl Acetate, Nordihydroguaiaretic Acid, Sodium Hyaluronate, Palmitoyl Tripeptide-38, Echium Plantagineum Seed Oil, Oenothera Biennis (Evening Primrose) Oil, Boerhavia Diffusa Root Extract, Phospholipids, Sphingolipids, Saccharide Isomerate, Citrus Aurantium Dulcis (Orange) Flower Extract, Glycolic Acid, Sodium PCA, Oleanolic Acid, PEG-60 Almond Glycerides, Hydrogenated Lecithin, Acrylates/Carbamate Copolymer, Bis-Ethylhexyl Hydroxydimethoxy Benzylmalonate, Coco-Caprylate/Caprate, Aluminum Hydroxide, Xanthan Gum, Citric Acid, Disodium EDTA, Hydroxypropyl Cyclodextrin, Sodium Citrate, Caprylyl Glycol, Isohexadecane, Polyhydroxystearic Acid, Sodium Hydroxide, Tromethamine, BHT, Polysorbate 60, Carbomer, Isopropyl Titanium Triisostearate, Sorbitan Isostearate, Stearic Acid, Benzyl Alcohol, Phenoxyethanol, Potassium Sorbate, Sodium Benzoate, Citrus Aurantium Dulcis (Orange) Oil, [+/- (May Contain): Titanium Dioxide (CI 77891), Mica (CI 77019), Iron Oxides (CI 77491, CI 77492, CI 77499)]
Questions?
Call 1-888-830-7546 Monday through Friday, 9:00 AM to 5:00 PM EST
PRINCIPAL DISPLAY PANEL - 50 ml Tube Carton - Light - Medium
NEW
SKINCARE
NO
CHEMICAL
FILTERS
SKINCARE
Dr Dennis Gross
instant radiance
sun defense
Sunscreen
Broad Spectrum
SPF 40
with sheer color
correction technology
1.7 fl oz/50ml
e
LIGHT – MEDIUM
Dr Dennis Gross
PRINCIPAL DISPLAY PANEL - 50 ml Tube Carton - Medium - Deep
NEW
SKINCARE
NO
CHEMICAL
FILTERS
SKINCARE
Dr Dennis Gross
instant radiance
sun defense
Sunscreen
Broad Spectrum
SPF 40
with sheer color
correction technology
1.7 fl oz/50ml
e
MEDIUM - DEEP
Dr Dennis Gross
Dr. Dennis Gross Skincare, LLC